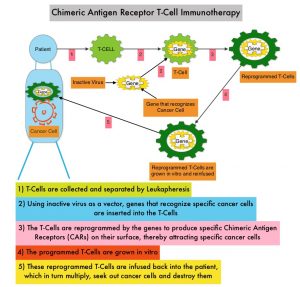
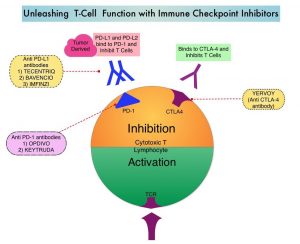
SUMMARY: Immunotherapy with PD-1/PD-L1 (Programmed Death-1/Programmed Death-Ligand 1) inhibitors, also called Immune Checkpoint Inhibitors (ICIs), has dramatically changed the treatment paradigm for patients with solid tumors, with significant improvement in outcomes. However, even among those with tumors expressing high PD-L1 expression and high Tumor Mutation Burden, not all patients benefit from Immunotherapy with ICIs. Therefore identifying biomarkers for patients likely to respond to ICI therapy, and predicting resistance is important and relevant, in selecting the appropriate patients for treatment with ICIs.
There is growing body of evidence on the role of inflammation in cancer biology, and systemic inflammatory response may have prognostic significance in different cancer types. Inflammatory process in various cancers imparts immunoresistance to ICIs, by activating oncogenic signaling pathways, there by promoting cancer growth and dissemination, with resulting poor outcomes.
More recently, attention has been focused on the predictive role of Platelet-Lymphocyte ratio (PLR) as an effective indicator of the severity of systemic inflammatory response. PLR is defined as the ratio of platelets to lymphocytes. Platelets and lymphocytes play multiple roles in the inflammatory response. Increased platelet count accelerates tumor progression by promoting neoangiogenesis and the production of adhesion molecules, whereas lymphocytes activate anti-tumor immunity by releasing a range of cytokines. Elevated PLR has been associated with poor prognosis in multiple solid tumors. In a meta-analysis of data from 12 related studies involving a total of 1340 patients, high PLR in cancer patients was associated with poor efficacy when treated with Immune Checkpoint Inhibitors, and poor prognosis. (https://doi.org/10.1016/j.intimp.2019.105957Get rights and content). Several other studies suggest that using PLR to predict the prognosis of cancer patients treated with immunotherapy remains controversial. The role of PLR in the prognosis of cancer patients treated with immunotherapy has thus remained unclear.
The present study was conducted to determine meaningful predictive factors for selecting patients with advanced Urothelial Carcinoma (UC) who might benefit clinically from treatment with Immune Checkpoint Inhibitor, KEYTRUDA® (Pembrolizumab). KEYTRUDA® is a fully humanized, Immunoglobulin G4, anti-PD-1, monoclonal antibody, that binds to the PD-1 receptor and blocks its interaction with ligands PD-L1 and PD-L2. It thereby reverses the PD-1 pathway-mediated inhibition of the immune response, and unleashes the tumor-specific effector T cells. The researchers retrospectively analyzed 54 patients who received treatment with KEYTRUDA® for Urothelial Carcinoma. Patient’s Hemoglobin, Albumin, Lymphocyte and Platelet (HALP) score, Neutrophil-to-Lymphocyte Ratio (NLR), and Platelet-to-Lymphocyte Ratio (PLR) were calculated as indices of systemic inflammatory response. The relationships between these scores and the initial tumor response or Overall Survival, as well as other clinicopathological factors, were then assessed.
It was noted that a high NLR and PLR were associated with a poor initial tumor response to KEYTRUDA®. A HALP score less than 30.05 and a PLR of 173.73 or more were associated with worse Overall Survival. In the multivariate analysis, a high PLR was a significant independent prognostic factor for unfavorable outcomes.
The authors concluded from this study that a high pretreatment Platelet-to-Lymphocyte Ratio may be a valuable indicator for choosing therapy other than KEYTRUDA® in patients with advanced Urothelial Carcinoma, and may be a potential biomarker for immunotherapy.
Platelet-to-Lymphocyte Ratio Predicts the Efficacy of Pembrolizumab in Patients With Urothelial Carcinoma. Kurashina R, Ando K, Inoue M, et al. Anticancer Research February 2022;42:1131-1136.