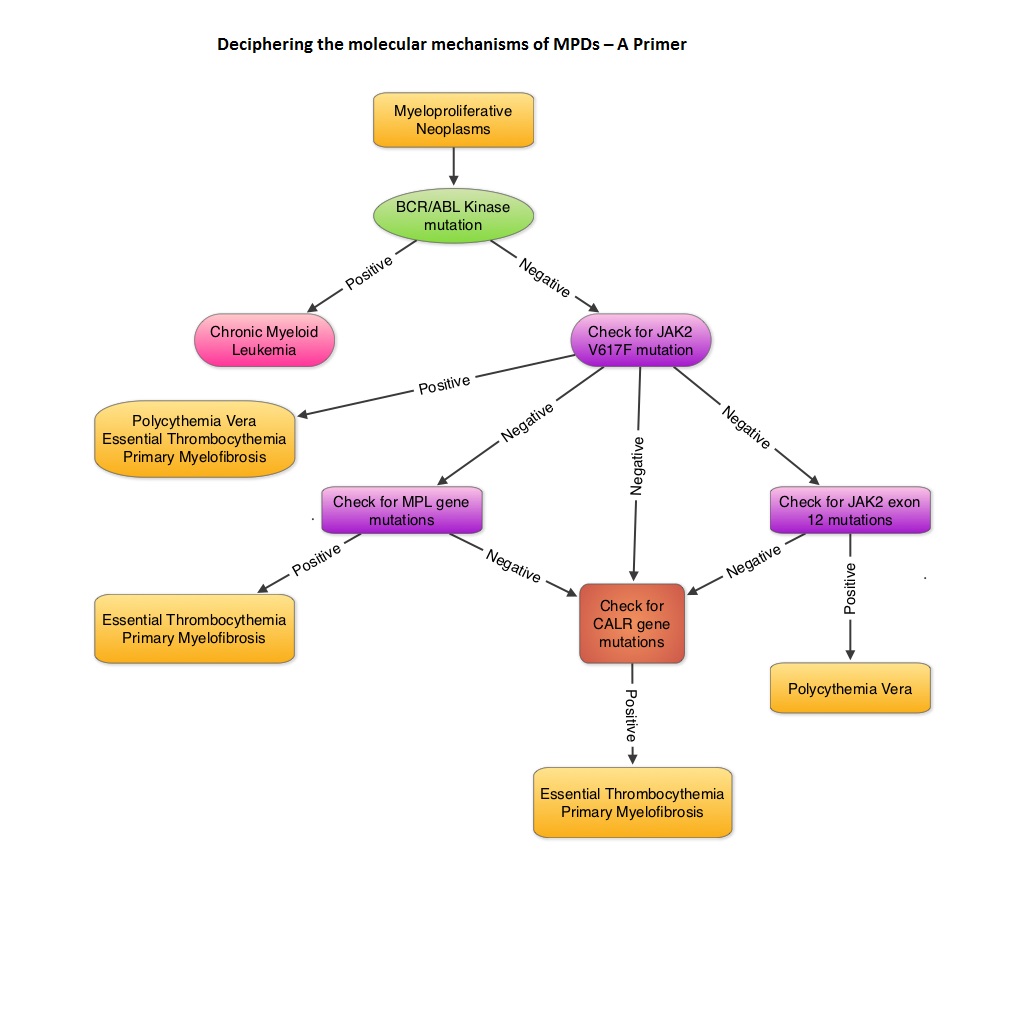
SUMMARY: MyeloProliferative Disorders (MPDs) are a group of clonal abnormalities associated with dysregulation of the multipotent hematopoietic stem cell (CD34). The most common MPDs include Chronic Myelogenous Leukemia (CML), Essential Thrombocythemia (ET), Polycythemia Vera (PV) and Primary MyeloFibrosis (PMF). These diseases have one or more shared features including hypercellular bone marrow with or without marrow fibrosis, overproduction of one or more blood cell lines, thrombotic and/or hemorrhagic tendencies, extramedullary hematopoiesis involving the liver or spleen, transformation to acute leukemia and molecular abnormalities. The Philadelphia chromosome which was discovered in 1960 results from a reciprocal translocation between the long arms of chromosomes 9 and 22, demonstrable in all hematopoietic precursors. This translocation results in the transfer of the Abelson (ABL) gene from chromosome 9 to join the Breakpoint Cluster Region (BCR) gene on chromosome 22. This fused BCR/ABL gene now on chromosome 22 is called the Philadelphia chromosome. The fusion gene is diagnostic of CML and is responsible for abnormal tyrosine kinase activity resulting in disordered myelopoiesis. The discovery of Philadelphia chromosome, established for the very first time, a direct link between chromosomal abnormalities and malignancy, subsequently leading to the development of targeted therapies. With regards to the other MPDs, majority of the patients with PV have activating JAK2 mutations such as JAK2V617F (95%) and if negative have JAK2 exon 12 mutations (5%). About 50% of the patients with ET and PMF have JAK2 mutations and about 5% have activating mutations in the thrombopoietin receptor gene (MPL). So, in the remaining approximately 45% of patients without these mutations, no specific molecular marker has been discovered. To address this group, the authors analyzed 1107 samples from patients with MPDs and were able to identify mutations in the gene encoding CALRETICULIN (CALR mutations), in certain groups of patients. They noted that CALR mutations are frameshift mutations never seen in patients with PV. They are however only demonstrable in the stem cells of ET or PMF patients, without JAK2 or MPL gene mutations. Therefore, in ET and PMF, CALR mutations and JAK2 and MPL mutations are mutually exclusive. Patients with CALR mutations have a more indolent course compared to those with JAK2 activating mutations, with a lower risk of thrombosis and longer overall survival. The authors concluded that CALR mutations may play an important early role in the development of MPDs and identifying this molecular marker may be relevant in the management of patients with ET and PMF. Klampfl T, Gisslinger H, Harutyunyan AS, et al. N Engl J Med 2013; 369:2379-2390