The FDA on December 9, 2022, approved TECENTRIQ® (Atezolizumab) for adult and pediatric patients 2 years of age and older with unresectable or metastatic Alveolar Soft Part Sarcoma (ASPS). TECENTRIQ® is a product of Genentech, Inc.
Tag: Soft Tissue Sarcomas – Trunk and Extremities
POMALYST® (Pomalidomide)
The FDA on May 14, 2020, expanded the indication of POMALYST® to include treating adult patients with AIDS-related Kaposi Sarcoma, after failure of highly active antiretroviral therapy, and Kaposi Sarcoma in adult patients who are HIV-negative. POMALYST® is a product of Celgene Corporation.
TAZVERIK® (Tazemetostat)
The FDA on January 23, 2020 granted accelerated approval to TAZVERIK® for adults and pediatric patients aged 16 years and older with metastatic or locally advanced epithelioid sarcoma, not eligible for complete resection. TAZVERIK® is a product of Epizyme, Inc.
FDA Approves PDGFRα Antagonist LARTRUVO® for Soft Tissue Sarcoma
SUMMARY: The FDA on October 19, 2016 granted accelerated approval to LARTRUVO® (Olaratumab) for the treatment of patients with Soft Tissue Sarcoma (STS), not amenable to curative treatment with radiotherapy or surgery, and with a histologic subtype for which an anthracycline containing regimen is appropriate. The American Cancer Society estimates that in 2016, about 12,310 new soft tissue sarcomas will be diagnosed in the United States and 4,990 patients will die of the disease. The most common types of Soft Tissue Sarcomas in adults are undifferentiated pleomorphic sarcoma (previously called Malignant Fibrous Histiocytoma), Liposarcoma, and Leiomyosarcoma. Patients with advanced Soft Tissue Sarcomas are often treated with a Doxorubicin based chemotherapy regimen and the median Overall Survival (OS) for those treated is 12-16 months.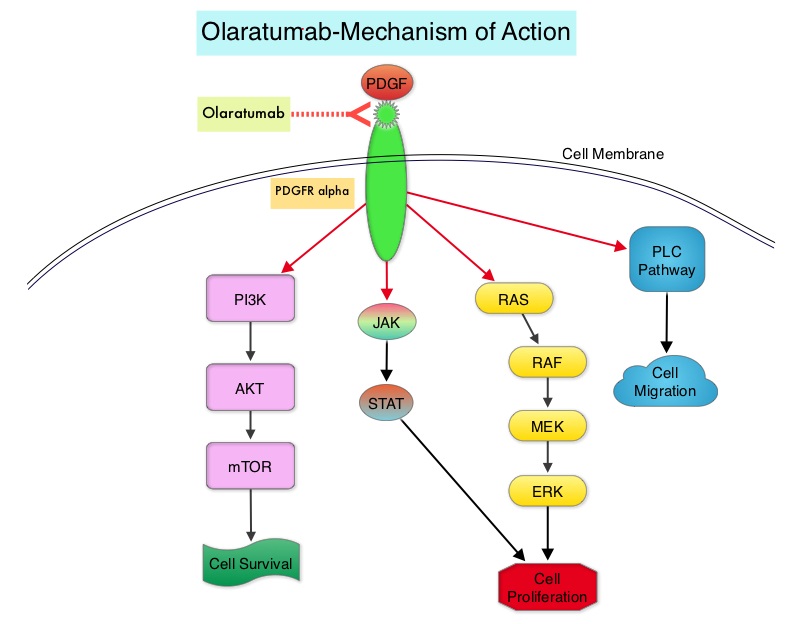
LARTRUVO® is a human IgG1 monoclonal antibody that binds to human PDGFRα with high affinity and blocks PDGFs (Platelet Derived Growth Factors) such as PDGF-AA, PDGF-BB, and PDGF-CC ligands, from binding to the receptor. Coexpression of PDGFRα and PDGFs, with associated autocrine-mediated cell growth, has been implicated in Sarcomas and Glioblastomas. Platelet Derived Growth Factor Receptor α (PDGFRα) is expressed in multiple tumor types and its aberrant activation may facilitate cancer development and spread.
The approval of LARTRUVO® was based on data from a randomized phase II study of Doxorubicin plus LARTRUVO® treatment, in patients with unresectable (locally advanced) or metastatic Soft Tissue Sarcoma (STS). In this pivotal trial, 133 patients with metastatic STS were randomized in a 1:1 ratio to receive LARTRUVO® plus Doxorubicin (N=66) or Doxorubicin alone (N=67). Enrolled patients had metastatic STS not amenable to curative treatment with surgery or radiotherapy, and a histologic type of sarcoma for which an anthracycline-containing regimen was appropriate, but had not been administered. LARTRUVO® was administered at 15 mg/kg as an IV infusion on days 1 and 8 of each 21-day cycle. All patients received doxorubicin 75 mg/m2 as an IV infusion on day 1 of each 21-day cycle for maximum of eight cycles. Single-agent LARTRUVO® was offered to patients in the Doxorubicin alone arm at the time of disease progression. The median patient age in the combination arm was 58.5 years and 88% of patients were positive for PDGFRα. Over a third of the enrolled patients had Leiomyosarcoma and over 25 different STS histologies were included in this study.
It was noted that there was a statistically significant improvement in Overall Survival (OS) for the combination treatment group with a median OS of 26.5 months compared to 14.7 months for those receiving Doxorubicin alone (HR=0.52; P<0.05). The median Progression Free Survival (independent review) was 8.2 months for patients in the combination group and 4.4 months for those receiving Doxorubicin alone (HR=0.74) and the Overall Response Rate (independent review) was 18% in the combination group and 8% in the Doxorubicin alone group. The most common (greater than or equal to 20%) side effects of treatment with LARTRUVO® were nausea, fatigue, neutropenia, musculoskeletal pain, mucositis, alopecia, vomiting, diarrhea, decreased appetite, abdominal pain, neuropathy, and headache. Infusion related reactions were seen in 13% of patients.
It was concluded that the combination of LARTRUVO® with Doxorubicin reduced the risk of death by 48% compared with Doxorubicin alone, for patients with advanced STS and is the first new therapy approved by the FDA for the initial treatment of Soft Tissue Sarcoma since the approval of Doxorubicin, more than 4 decades ago. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Tap WD, Jones RL, Van Tine BA, et al. Lancet. 2016;388:488-497
LARTRUVO® (Olaratumab)
The FDA on October 19, 2016 granted accelerated approval to LARTRUVO® for the treatment of patients with Soft Tissue Sarcoma (STS) not amenable to curative treatment with radiotherapy or surgery and with a histologic subtype for which an Anthracycline-containing regimen is appropriate. LARTRUVO® is a product of Eli Lilly and Company.
HALAVEN® (Eribulin)
The FDA on January 28, 2016 approved HALAVEN® for the treatment of patients with unresectable or metastatic Liposarcoma who have received a prior Anthracycline-containing regimen. HALAVEN® is a product of Eisai Co., Ltd.
YONDELIS® – A Marine-Derived Drug for Soft Tissue Sarcomas
SUMMARY: The FDA approved the first “Liquid Biopsy” test on June 1, 2016 for the detection of exon 19 deletions or exon 21 (L858R) substitution mutations in the Epidermal Growth Factor Receptor (EGFR) gene. On the heels of this approval, Zill and colleagues reported the results of the largest liquid biopsy study ever conducted thus far. It has been well established that treatment with EGFR TKIs results in superior outcomes, for patients with tumors harboring exon 19 deletions and exon 21 mutations. The application of precision medicine with targeted therapy requires detection of molecular abnormalities in a tumor specimen, following progression or recurrence. Archived biopsy specimens may not be helpful, as it is important to identify additional mutations in the tumor at the time of recurrence or progression, in order to plan appropriate therapy. Further, recurrent tumors may be inaccessible for a safe biopsy procedure or the clinical condition of the patient may not permit a repeat biopsy. Additionally, the biopsy itself may be subject to sampling error due to tumor heterogeneity. Genotyping cell free DNA in the plasma, also called liquid biopsy, can potentially overcome the shortcomings of repeat biopsies and tissue genotyping, allowing the detection of many more targetable gene mutations, thus resulting in better evaluation of the tumor genome landscape.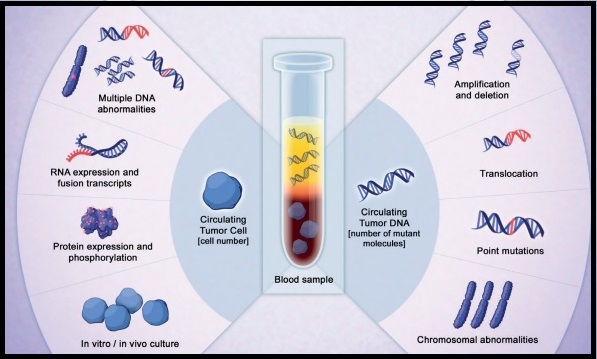
The authors in this study utilized Next Generation Sequencing (NGS) of circulating tumor DNA (ctDNA), isolated from plasma specimens (liquid biopsy specimens) of 15,191 patients of whom 37% had advanced lung cancer, 14% had breast cancer, 10% had colorectal cancer and 39% had other malignancies. Seventy genes were targeted and accuracy of ctDNA sequencing was assessed by comparing with matched tissue tests for 386 patients and frequencies of somatic ctDNA alterations per gene were compared to those previously described in tissue sequencing projects such as data from The Cancer Genome Atlas (TCGA).
It was noted that the ctDNA mutation patterns were highly concordant with tissue analysis as reported by the TCGA. The overall accuracy of ctDNA sequencing in comparison with matched tissue tests was 87% and the accuracy increased to 98% when blood and tumor were collected less than six months apart. Pearson Correlation between sets of data is a measure of how well these sets are related. Between 0.5 and 1.0 is considered high correlation. Pearson correlation for TP53 gene was 0.94, for KRAS was 0.99 and for PIK3CA was 0.99.
The researchers commented on the clinical outcome benefits using liquid biopsy, in four distinct groups:
1) Testing for actionable mutations (ALK fusion, EGFR or BRAF activating mutations in lung; ERBB2 amplification in gastric cancer) in cases with insufficient tissue quantity.
2) Testing for actionable resistance mutations (MET amplification or EGFR T790M in lung cancer), at the time of progression.
3) Genomic evolution upon progression such as ERBB2-amplified metastatic breast cancer in patients with triple negative primary tumor.
4) Tumors with genotypes that need more extensive driver mutation testing such as BRAF V600E in lung.
The authors concluded that there is a high correlation between ctDNA plasma samples and tissue testing with the exception of resistance mutations such as EGFR T790M mutation which evolve while on anti-EGFR inhibitor therapy and consequently may not correlate with the TCGA, probably because patients in the tissue-based population had not yet received the anti-EGFR inhibitor therapy that promotes the mutation. Patients who received treatment based on ctDNA findings also experienced better clinical outcomes. Zill OA, Mortimer S, Banks KC, et al Somatic genomic landscape of over 15,000 patients with advanced-stage cancer from clinical next-generation sequencing analysis of circulating tumor DNA. J Clin Oncol. 2016;34(suppl; abstr LBA11501).
FDA Approves HALAVEN® for Advanced Liposarcoma
SUMMARY: The FDA on January 28, 2016, approved HALAVEN® (Eribulin) for the treatment of patients with unresectable or metastatic Liposarcoma, who have received a prior Anthracycline-containing regimen. The American Cancer Society's estimates that in the United States, approximately 11,930 new Soft Tissue Sarcomas were diagnosed in 2015 and 4,870 patients died of the disease. The most common types of sarcoma in adults are, Undifferentiated Pleomorphic Sarcoma ( Malignant Fibrous Histiocytoma), Liposarcoma, and Leiomyosarcoma. Leiomyosarcomas often present as abdominal sarcomas, whereas Liposarcomas and Undifferentiated Pleomorphic Sarcomas develop in the extremities. There are close to 50 different types of Soft Tissue Sarcomas. Liposarcomas are malignant tumors of the adipose tissue.
The approval of HALAVEN® was based on an open-label, randomized, multicenter, phase III trial in which 446 patients with unresectable, locally advanced or metastatic Liposarcoma or Leiomyosarcoma were randomly assigned in a 1:1 ratio to receive either HALAVEN® (N=225) or Dacarbazine (N=221). Eligible patients had received at least two prior systemic chemotherapies (one of which must have included an Anthracycline) and had disease progression within 6 months of randomization. Randomized patients received either HALAVEN® 1.4 mg/m2 on days 1 and 8 of a 21-day cycle or Dacarbazine 850 mg/m2, 1000 mg/m2, or 1200 mg/m2 chosen by the investigator prior to randomization, on day 1 of a 21-day treatment cycle. Treatment was continued until disease progression or unacceptable toxicity. Patients were stratified by histology (Liposarcoma vs. Leiomyosarcoma) and 68% (N=303) had Leiomyosarcoma and 32% (N=143) had Liposarcoma. Majority of the patients had received more than two prior systemic chemotherapies. The median age was 56 years. The primary endpoint of this study was Overall Survival and secondary endpoints included Progression Free Survival and Safety.
The trial met its primary endpoint with a statistically significant improvement in Overall Survival (OS) in the HALAVEN® group compared to the Dacarbazine group. The median OS was 13.5 months in the HALAVEN® arm and 11.3 months in the Dacarbazine arm (HR=0.75; P=0.011). There was no improvement noted in the Progression Free Survival (PFS) or Objective Response Rates in the overall study population. In the pre-planned, exploratory subgroup analyses of OS and PFS, the benefit with HALAVEN® treatment was limited to the subgroup of patients with Liposarcoma (N=143), with a median OS of 15.6 versus 8.4 months for the Dacarbazine group (HR=0.51). There was no treatment benefit with HALAVEN® compared to Dacarbazine treatment, for patients with Leiomyosarcoma (median OS of 12.8 vs 12.3 months; HR=0.90 and median PFS of 2.2 vs 2.6 months; HR=1.05).
The most common adverse reactions associated with HALAVEN® treatment were fever, fatigue, nausea, alopecia, constipation, peripheral neuropathy and neutropenia. Thrombocytopenia was more frequent in the Dacarbazine group than HALAVEN® group. It was concluded that HALAVEN® significantly improves Overall Survival in patients with advanced, pretreated Liposarcoma and is the first drug approved for this patient population. Schöffski P, Maki RG, Italiano A, et al. Randomized, open-label, multicenter, phase III study of eribulin versus dacarbazine in patients (pts) with leiomyosarcoma (LMS) and adipocytic sarcoma (ADI). J Clin Oncol. 2015;(suppl; abstr LBA10502).
YONDELIS® (Trabectedin)
The FDA on October 23, 2015 approved YONDELIS®) for the treatment of patients with unresectable or metastatic Liposarcoma or Leiomyosarcoma who have received a prior Anthracycline-containing regimen. YONDELIS® injection is a product of Janssen Biotech, Inc.
FDA Approves YONDELIS® for Soft Tissue Sarcomas
SUMMARY: The U.S. FDA approved YONDELIS® (Trabectedin) for the treatment of patients with unresectable or metastatic Liposarcoma or Leiomyosarcoma, who have received a prior Anthracycline-containing regimen. Soft Tissue Sarcomas are a heterogeneous group of tumors of mesenchymal origin with over 50 different histological variants. The American Cancer Society estimates that in 2015, about 11,930 new soft tissue sarcomas will be diagnosed in the United States and 4,870 patients will die of the disease. The most common types of Soft Tissue Sarcomas in adults are undifferentiated pleomorphic sarcoma (previously called Malignant Fibrous Histiocytoma), Liposarcoma, and Leiomyosarcoma. Chemotherapy is the standard of care for advanced Soft Tissue Sarcomas. Following first line therapy with Anthracycline or Ifosfamide based chemotherapy regimens, Response Rates are low and second line treatment options are limited. YONDELIS® (Trabectedin) originally isolated from the Caribbean sea sponge (Ecteinascidia turbinate) is a synthetic alkaloid that binds to the minor groove of DNA and induces apoptosis by damaging the DNA.
Based on promising phase II trials, a randomized, open-label, multicenter, phase III trial was conducted, in which 518 patients with unresectable, locally advanced or metastatic Liposarcoma or Leiomyosarcomas were randomly assigned in a 2:1 to receive either YONDELIS® or Dacarbazine. YONDELIS® was dosed at 1.5 mg/m2, administered as an intravenous infusion over 24 hours (N=345) and Dacarbazine was dosed at 1000 mg/m2 administered as an intravenous infusion over 20 to 120 minutes (N=173) and the treatment was given once every 3 weeks. The median age was 56 years and enrolled patients were heavily pretreated and had received prior Anthracycline containing chemotherapy regimens. Close to 90% of the patients had received at least two prior lines of chemotherapy. Patients were stratified by Soft Tissue Sarcoma subtype (Leiomyosarcoma vs Liposarcoma), ECOG performance status, and number of prior chemotherapy regimens. The primary end point was Overall Survival (OS) and secondary end points included Progression Free Survival (PFS), Time To Progression, Objective Response Rate, Duration of Response, as well as safety.
This study demonstrated a statistically significant improvement in PFS in the YONDELIS® group with a 45% reduction in the risk of disease progression or death compared with Dacarbazine (HR= 0.55; P<0.001). The median PFS was 4.2 and 1.5 months in the YONDELIS® and Dacarbazine groups, respectively. This benefit was noted in patients with both Leiomyosarcoma and Liposarcoma. The greatest benefit in median PFS (5.6 months vs 1.5 months with YONDELIS® vs Dacarbazine, respectively) was noted in the myxoid or round cell Liposarcomas, which are considered as translocation-related sarcomas. This additional benefit can be explained based on the direct inhibition by YONDELIS®, of the chimeric FUS-CHOP translocation-generated oncoprotein, which regulates transcriptional activity in myxoid or round cell Liposarcomas. There was however no significant difference in the median Overall Survival between YONDELIS® and Dacarbazine (12.9 months vs 12.4 months, P=0.37). This may be due to crossover of close to 30% of patients who progressed on Dacarbazine to Receptor Tyrosine Kinase Inhibitor, VOTRIENT® (Pazopanib), which was approved in the U.S. during the course of this study. Most of patients who benefited from YONDELIS®, experienced stable disease as their best response for longer durations, compared to those with Dacarbazine (51% vs 35%).
The most common adverse reactions (20% or more) with YONDELIS® were nausea, fatigue, vomiting, constipation, decreased appetite, diarrhea, peripheral edema, dyspnea, and headache. The most common grade 3 to 4 adverse effects were myelosuppression and transient elevation of transaminases. The authors concluded that YONDELIS® significantly improves Progression Free Survival with superior disease control compared to Dacarbazine, in patients with heavily pretreated, advanced Liposarcoma and Leiomyosarcoma. Efficacy and Safety of Trabectedin or Dacarbazine for Metastatic Liposarcoma or Leiomyosarcoma After Failure of Conventional Chemotherapy: Results of a Phase III Randomized Multicenter Clinical Trial. Demetri GD, von Mehren M, Jones RL, et al. J Clin Oncol, doi: 10.1200/JCO.2015.62.4734.
