SUMMARY: Chemotherapy Induced Nausea and Vomiting (CINV) is one of the most common adverse effects of chemotherapy and is experienced by about 80% of patients receiving chemotherapy. The development of effective antiemetic agents has facilitated the administration of majority of the chemotherapy agents in an outpatient setting avoiding hospitalization.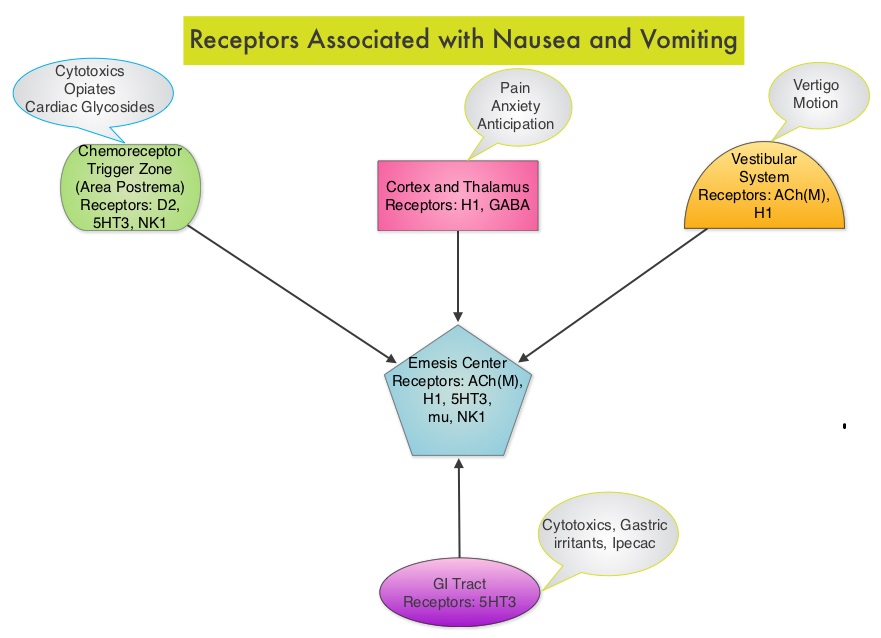 Acute CINV begins within the first 24 hours following chemotherapy administration, with most patients experiencing symptoms within the first four hours of treatment whereas delayed nausea and vomiting occurs more than 24 hours after chemotherapy administration and can persist for several days. Delayed CINV is often underestimated and a third of the patients receiving chemotherapy may experience delayed nausea and vomiting without prior acute nausea or vomiting. Acute nausea and vomiting is dependent on serotonin (5-hydroxytryptamine-5HT3) and its receptors. 5-HT3 receptors are located on vagal afferent pathway, which in turn activates the vomiting center to initiate the vomiting reflex. 5-HT3 receptors are located peripherally on the nerve endings of the vagus and centrally in the Chemoreceptor Trigger Zone of the area Postrema. Chemotherapeutic agents produce nausea and vomiting by stimulating the release of serotonin from the enterochromaffin cells of the small intestine. Delayed nausea and vomiting is associated with the activation of Neurokinin 1 (NK1) receptors by substance P. NK1 receptors are broadly distributed in the central and peripheral nervous systems. Netupitant inhibits substance P mediated responses. ALOXI® (Palonosetron) is a second generation 5-HT3 antagonist and has a 100 fold higher binding affinity to 5-HT3 receptor than other 5-HT3 receptor antagonists. AKYNZEO® (300 mg Netupitant/0.5 mg Palonosetron) is an oral, fixed combination product of Netupitant, a substance P/Neurokinin 1 (NK1) receptor antagonist, and ALOXI®, a serotonin (5- HT3) receptor antagonist.
Acute CINV begins within the first 24 hours following chemotherapy administration, with most patients experiencing symptoms within the first four hours of treatment whereas delayed nausea and vomiting occurs more than 24 hours after chemotherapy administration and can persist for several days. Delayed CINV is often underestimated and a third of the patients receiving chemotherapy may experience delayed nausea and vomiting without prior acute nausea or vomiting. Acute nausea and vomiting is dependent on serotonin (5-hydroxytryptamine-5HT3) and its receptors. 5-HT3 receptors are located on vagal afferent pathway, which in turn activates the vomiting center to initiate the vomiting reflex. 5-HT3 receptors are located peripherally on the nerve endings of the vagus and centrally in the Chemoreceptor Trigger Zone of the area Postrema. Chemotherapeutic agents produce nausea and vomiting by stimulating the release of serotonin from the enterochromaffin cells of the small intestine. Delayed nausea and vomiting is associated with the activation of Neurokinin 1 (NK1) receptors by substance P. NK1 receptors are broadly distributed in the central and peripheral nervous systems. Netupitant inhibits substance P mediated responses. ALOXI® (Palonosetron) is a second generation 5-HT3 antagonist and has a 100 fold higher binding affinity to 5-HT3 receptor than other 5-HT3 receptor antagonists. AKYNZEO® (300 mg Netupitant/0.5 mg Palonosetron) is an oral, fixed combination product of Netupitant, a substance P/Neurokinin 1 (NK1) receptor antagonist, and ALOXI®, a serotonin (5- HT3) receptor antagonist.  Taking advantage of the different mechanisms of action and synergy between these two agents, a randomized, double-blind, multinational study was conducted, comparing AKYNZEO® with ALOXI® in chemotherapy naive patients receiving anthracycline based chemotherapy regimens. One thousand four hundred and fifty five (N=1455) were randomized to receive either AKYNZEO® or ALOXI® and both groups received oral Dexamethasone as a part of their antiemetic regimen. The primary endpoint was complete response (CR) defined as no emesis, no rescue medication needed and no significant nausea. AKYNZEO® maintained superiority over ALOXI® for overall (0-120 hours) complete response and also maintained superiority over multiple chemotherapy cycles (P < 0.0001). The most common side effects for AKYNZEO® were headache, fatigue and constipation. The authors concluded that AKYNZEO®, by targeting dual antiemetic pathways, significantly improved chemotherapy induced nausea and vomiting compared to ALOXI® alone and this benefit was maintained over multiple cycles of moderately emetogenic chemotherapy. AKYNZEO® capsule can be administered as a single dose, one hour prior to the start of chemotherapy. Aapro MS, Karthaus M, Schwartzberg LS, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 9502)</s
Taking advantage of the different mechanisms of action and synergy between these two agents, a randomized, double-blind, multinational study was conducted, comparing AKYNZEO® with ALOXI® in chemotherapy naive patients receiving anthracycline based chemotherapy regimens. One thousand four hundred and fifty five (N=1455) were randomized to receive either AKYNZEO® or ALOXI® and both groups received oral Dexamethasone as a part of their antiemetic regimen. The primary endpoint was complete response (CR) defined as no emesis, no rescue medication needed and no significant nausea. AKYNZEO® maintained superiority over ALOXI® for overall (0-120 hours) complete response and also maintained superiority over multiple chemotherapy cycles (P < 0.0001). The most common side effects for AKYNZEO® were headache, fatigue and constipation. The authors concluded that AKYNZEO®, by targeting dual antiemetic pathways, significantly improved chemotherapy induced nausea and vomiting compared to ALOXI® alone and this benefit was maintained over multiple cycles of moderately emetogenic chemotherapy. AKYNZEO® capsule can be administered as a single dose, one hour prior to the start of chemotherapy. Aapro MS, Karthaus M, Schwartzberg LS, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 9502)</s
Tag: Palliative and Supportive Care
Prevention of Pegfilgrastim-Induced Bone Pain A Phase III Double-Blind Placebo-Controlled Randomized Clinical Trial of the University of Rochester Cancer Center Clinical Community Oncology Program Research Base
SUMMARY:Bone pain related to NEULASTA® (Pegfilgrastim) has been reported to 25%-60% of patients in various clinical trials. In this randomized placebo controlled clinical trial, 510 patients were randomly assigned to receive either Naproxen 500 mg PO BID or placebo, for 5-8 days following NEULASTA®. Naproxen reduced the proportion of pain, patients reported, by 10% (71% vs 61%, P=0.02) and severe pain was reduced by 8% (27% vs 19%, P=0.05). Naproxen also reduced the overall pain incidence (P=0.02) and duration of pain (P=0.009). The authors concluded that Naproxen 500mg PO BID is effective in modestly reducing the incidence and severity of NEULASTA® induced bone pain. The adverse effects of Naproxen, has to be taken into consideration before prescribing this agent for bone pain. Kirshner JJ, Heckler CE, Janelsins MC, et al. J Clin Oncol 2012;30:1974-1979.
The use of olanzapine versus metoclopramide for the treatment of breakthrough chemotherapy-induced nausea and vomiting (CINV) in patients receiving highly emetogenic chemotherapy
SUMMARY: Breakthrough Chemotherapy Induced Nausea and Vomiting (CINV) is encountered in 30-40% of patients, in spite of treatment with prophylactic antiemetics. ZYPREXA® (Olanzapine) is a FDA approved antipsychotic agent. In this double blind, randomized, phase III trial, chemotherapy naïve patients receiving highly emetogenic chemotherapy who had experienced breakthrough CINV were randomized to receive either ZYPREXA® 10 mg orally daily for three days or REGLAN® (Metoclopramide) 10mg orally TID for three days. They were then monitored for 72 hours. During this 72 hour observation period, 71% patients receiving ZYPREXA® had no emesis compared to 32% in the group receiving REGLAN® (P<0.01). With regards to nausea, 67% of patients in the ZYPREXA® group did not experience nausea, compared to 24% in the REGLAN® group (P<0.01). This new treatment option with ZYPREXA® may help a significant number of patients who experience breakthrough CINV. Navari RM, Nagy CK, Gray SE. J Clin Oncol. 2012; 30 (suppl; abstr 9064).
