SUMMARY: The FDA on September 15, 2023 approved Momelotinib (OJJAARA®) for the treatment of adult patients with intermediate or high-risk myelofibrosis, including Primary myelofibrosis or Secondary myelofibrosis, and anemia. Myelofibrosis is a MyeloProliferative Neoplasm (MPN) characterized by ineffective hematopoiesis, progressive fibrosis of the bone marrow and potential for leukemic transformation. It affects approximately 25,000 patients in the United States. This stem cell disorder is Philadelphia Chromosome negative and manifestations include anemia, splenomegaly and related symptoms such as abdominal distension and discomfort with early satiety. Cytokine driven debilitating symptoms such as fatigue, fever, night sweats, weight loss, pruritus and bone or muscle pain can further impact an individual’s quality of life. Myelofibrosis can be Primary (PMF) or Secondary to Polycythemia Vera (PV) or Essential Thrombocythemia (ET).
The JAK-STAT signaling pathway has been implicated in the pathogenesis of myelofibrosis. This pathway normally is responsible for passing information from outside the cell through the cell membrane to the DNA in the nucleus, for gene transcription. Janus Kinase (JAK) family of tyrosine kinases are cytoplasmic proteins and include JAK1, JAK2, JAK3 and TYK2. JAK1 helps propagate the signaling of inflammatory cytokines whereas JAK2 is essential for growth and differentiation of hematopoietic stem cells. These tyrosine kinases mediate cell signaling by recruiting STATs (Signal Transducer and Activator of Transcription), with resulting modulation of gene expression.
In patients with MPN, the aberrant myeloproliferation is the result of dysregulated JAK2-STAT signaling as well as excess production of inflammatory cytokines associated with this abnormal signaling. These cytokines contribute to the systemic symptoms often reported by patients with myelofibrosis, in addition to bone marrow fibrosis, and clonal proliferation resulting in extramedullary hematopoiesis and splenomegaly. JAK2 mutations such as JAK2 V617F are seen in approximately 60% of the patients with PMF and ET and 95% of patients with PV. Unlike CML where the BCR-ABL fusion gene triggers the disease, JAK2 mutations are not initiators of the disease and are not specific for MPN. Further, several other genetic events may contribute to the abnormal JAK2-STAT signaling.
Chronic inflammation also drives hyperactivation of ACVR1 (Activin A receptor type 1), elevated hepcidin, dysregulated iron metabolism, and anemia of myelofibrosis. Approximately 40% of patients have moderate to severe anemia at the time of diagnosis of myelofibrosis, and almost all patients develop anemia over the course of the disease.
Momelotinib is a first-in-class inhibitor of three signaling pathways, Activin A receptor type 1 (ACVR1) as well as JAK1 and JAK2. Inhibition of ACVR1 leads to a decrease in circulating hepcidin, which is elevated in myelofibrosis and contributes to anemia, whereas inhibition of JAK1 and JAK2 may improve constitutional symptoms and splenomegaly.
The FDA approval was based on the findings from the pivotal Phase III MOMENTUM trial, and data from a subpopulation of adult patients with anemia enrolled in the SIMPLIFY-1 Phase III trial.
MOMENTUM is an ongoing Phase III, global, multicentre, randomized, double-blind study, conducted to evaluate the safety and efficacy of Momelotinib compared to Danazol, in patients with myelofibrosis who were symptomatic and anemic, and had been previously treated with an approved JAK inhibitor. This study was designed to evaluate the benefit of Momelotinib in reducing the key manifestations of myelofibrosis including constitutional symptoms, blood transfusion requirements due to anemia and splenomegaly. In this study, 195 eligible patients, with a confirmed diagnosis of Primary myelofibrosis, post-Polycythemia Vera (PV) myelofibrosis, or post–Essential Thrombocytopenia (ET) myelofibrosis were randomized 2:1 to receive Momelotinib 200 mg orally daily along with a placebo (N=130) or Danazol 600 mg orally daily along with a placebo (N=65). Enrolled patients were symptomatic with a Total Symptom Score (TSS) of at least 10, hemoglobin less than 10 gm/dL and platelet count 25,000/L or more. The median age was 70 years, approximately 50% of patients were Transfusion Dependent requiring 4 or more units of RBC transfusions in the 8 weeks before randomization and approximately 35% were Transfusion Requiring, who required RBC transfusions but did not meet the criteria for Transfusion Dependence. The Primary end point was achievement of a Myelofibrosis Symptom Assessment Form (MFSAF v4.0) TSS reduction of 50% or more at week 24 compared to baseline. Patients in the Danazol group were allowed to cross over to Momelotinib after week 24. It was noted that at week 24, 25% of patients in the Momelotinib group experienced a TSS reduction of 50% or more, compared with 9% in the Danazol group (P=0.0095). At week 24, 31% of patients who received Momelotinib achieved Transfusion Independence, compared with 20% in the Danazol group. Further, 39% of patients in the Momelotinib group experienced a splenic volume response of at least 25% by week 24, compared to 6% in the Danazol group (P<0.0001).
SIMPLIFY-1 was a multicentre, randomized, double-blind, Phase III study in which the efficacy and safety of Momelotinib was compared Ruxolitinib in patients with myelofibrosis who had not received prior treatment with a JAK inhibitor. In this study 432 eligible patients (N=432) received Momelotinib 200 mg orally once daily or Ruxolitinib (JAKAFI®) at an adjusted dose twice daily for 24 weeks. Subsequently, those in the Ruxolitinib arm were able to switch to open-label Momelotinib. Safety and efficacy results for SIMPLIFY-1 were based upon a subset of patients with anemia (hemoglobin less than 10 g/dL) at baseline. The efficacy of Momelotinib in the treatment of patients with myelofibrosis in SIMPLIFY-1 was based on spleen volume response (reduction by 35% or greater). In this study, Transfusion Independence was significantly improved at week 24 with Momelotinib compared to Ruxolitinib. Results in this study were mixed and Momelotinib offered less symptom control than Ruxolitinib, but there was comparable spleen volume reduction and a potential benefit in terms of anemia.
These findings support the potential use of Momelotinib as an effective treatment in patients with myelofibrosis, particularly in patients with anemia and thrombocytopenia, thus fulfilling an unmet need for these patients.
Ojjaara (momelotinib) approved in the US as the first and only treatment indicated for myelofibrosis patients with anaemia. News release. GSK. September 15, 2023. Accessed September 15, 2023. https://www.gsk.com/en-gb/media/press-releases/ojjaara-momelotinib-approved-in-the-us-as-the-first-and-only-treatment-indicated-for-myelofibrosis-patients-with-anaemia/

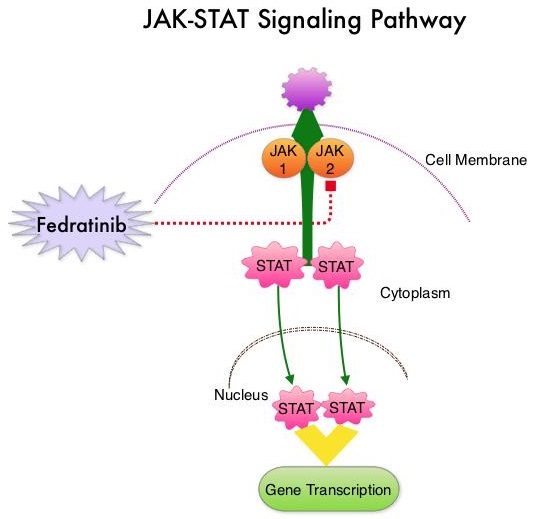
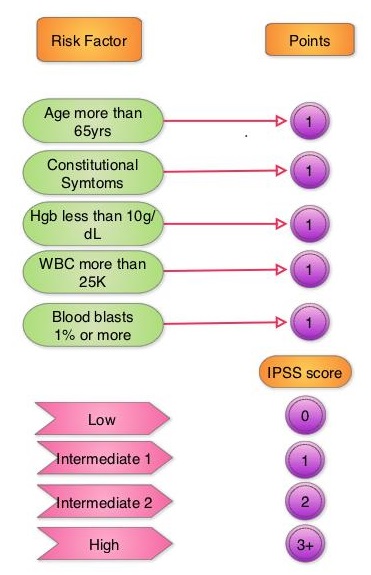 The approval of INREBIC®was based on findings from the
The approval of INREBIC®was based on findings from the 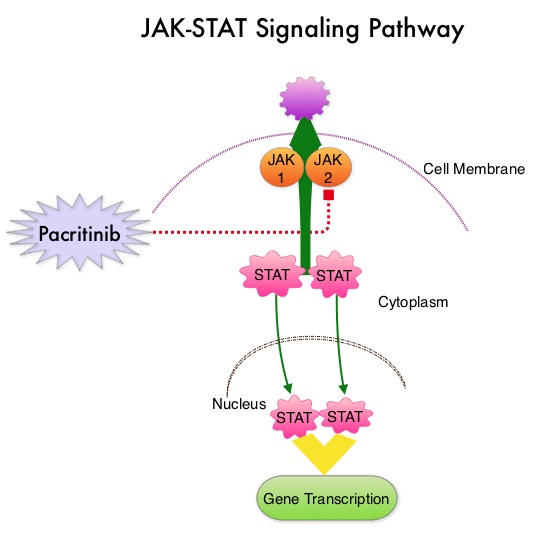
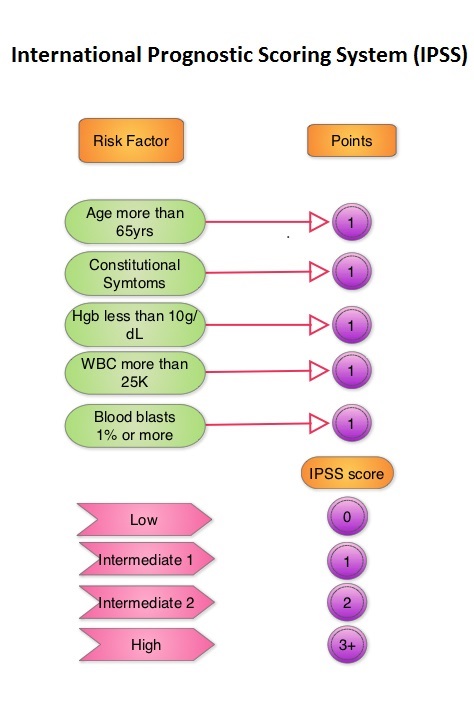 JAKAFI® (Ruxolitinib) is a potent JAK1 and JAK2 inhibitor approved by the FDA in 2011 to treat intermediate or high-risk Myelofibrosis. It is however not indicated for patients with platelet counts under 50,000/μl, and this group represents approximately one third of Myelofibrosis patients and have limited or no treatment options. Previously published PERSIST-1 trial showed that Pacritinib significantly reduced Spleen Volume and Myelofibrosis associated symptoms, in patients with low platelet count, when compared to Best Available Therapy (excluding JAKAFI®).
JAKAFI® (Ruxolitinib) is a potent JAK1 and JAK2 inhibitor approved by the FDA in 2011 to treat intermediate or high-risk Myelofibrosis. It is however not indicated for patients with platelet counts under 50,000/μl, and this group represents approximately one third of Myelofibrosis patients and have limited or no treatment options. Previously published PERSIST-1 trial showed that Pacritinib significantly reduced Spleen Volume and Myelofibrosis associated symptoms, in patients with low platelet count, when compared to Best Available Therapy (excluding JAKAFI®).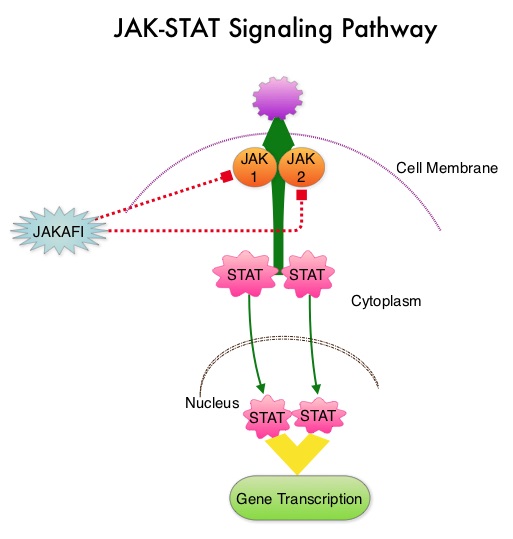 The JAK-STAT signaling pathway has been implicated in the pathogenesis of Myelofibrosis. This pathway normally is responsible for passing information from outside the cell through the cell membrane to the DNA in the nucleus for gene transcription. Janus Kinase (JAK) family of tyrosine kinases are cytoplasmic proteins and include JAK1, JAK2, JAK3 and TYK2. JAK1 helps propagate the signaling of inflammatory cytokines whereas JAK2 is essential for growth and differentiation of hematopoietic stem cells. These tyrosine kinases mediate cell signaling by recruiting STAT’s (Signal Transducer and Activator of Transcription), with resulting modulation of gene expression. In patients with MPN, the aberrant myeloproliferation is the result of dysregulated JAK2-STAT signaling as well as excess production of inflammatory cytokines associated with this abnormal signaling. These cytokines contribute to the symptoms often reported by patients with MF. JAK2 mutations such as JAK2 V617F are seen in approximately 60% of the patients with PMF and ET and 95% of patients with PV. Unlike CML where the BCR-ABL fusion gene triggers the disease, JAK2 mutations are not initiators of the disease and are not specific for MPN. Further, several other genetic events may contribute to the abnormal JAK2-STAT signaling.
The JAK-STAT signaling pathway has been implicated in the pathogenesis of Myelofibrosis. This pathway normally is responsible for passing information from outside the cell through the cell membrane to the DNA in the nucleus for gene transcription. Janus Kinase (JAK) family of tyrosine kinases are cytoplasmic proteins and include JAK1, JAK2, JAK3 and TYK2. JAK1 helps propagate the signaling of inflammatory cytokines whereas JAK2 is essential for growth and differentiation of hematopoietic stem cells. These tyrosine kinases mediate cell signaling by recruiting STAT’s (Signal Transducer and Activator of Transcription), with resulting modulation of gene expression. In patients with MPN, the aberrant myeloproliferation is the result of dysregulated JAK2-STAT signaling as well as excess production of inflammatory cytokines associated with this abnormal signaling. These cytokines contribute to the symptoms often reported by patients with MF. JAK2 mutations such as JAK2 V617F are seen in approximately 60% of the patients with PMF and ET and 95% of patients with PV. Unlike CML where the BCR-ABL fusion gene triggers the disease, JAK2 mutations are not initiators of the disease and are not specific for MPN. Further, several other genetic events may contribute to the abnormal JAK2-STAT signaling. The authors now reported the final long term efficacy and safety results after 5 years of treatment with JAKAFI® in the COMFORT-I study. In COMFORT-I study, 309 intermediate or high risk patients were randomized to receive either JAKAFI® (N=155) or Placebo (N=154). The Primary end point was a 35% or more reduction in spleen size at 24 weeks. The preplanned 5- year analysis occurred when all patients reached the 5-year visit or discontinued treatment. Patients in the placebo group could crossover to the JAKAFI® group after the primary analysis (when all patients completed week 24) or at any time if they had pre-specified worsening of splenomegaly. Of the 154 patients randomized to placebo, 111 patients crossed over to the JAKAFI® group and the median time to crossover was 41 weeks.
The authors now reported the final long term efficacy and safety results after 5 years of treatment with JAKAFI® in the COMFORT-I study. In COMFORT-I study, 309 intermediate or high risk patients were randomized to receive either JAKAFI® (N=155) or Placebo (N=154). The Primary end point was a 35% or more reduction in spleen size at 24 weeks. The preplanned 5- year analysis occurred when all patients reached the 5-year visit or discontinued treatment. Patients in the placebo group could crossover to the JAKAFI® group after the primary analysis (when all patients completed week 24) or at any time if they had pre-specified worsening of splenomegaly. Of the 154 patients randomized to placebo, 111 patients crossed over to the JAKAFI® group and the median time to crossover was 41 weeks.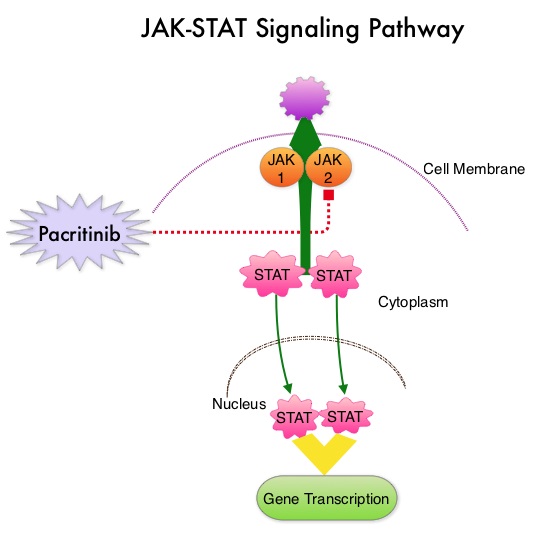 The JAK-STAT signaling pathway has been implicated in the pathogenesis of Myelofibrosis. This pathway normally is responsible for passing information from outside the cell through the cell membrane to the DNA in the nucleus, for gene transcription. Janus Kinase (JAK) family of tyrosine kinases are cytoplasmic proteins and include JAK1, JAK2, JAK3 and TYK2. JAK1 helps propagate the signaling of inflammatory cytokines whereas JAK2 is essential for growth and differentiation of hematopoietic stem cells. These tyrosine kinases mediate cell signaling by recruiting STAT’s (Signal Transducer and Activator of Transcription), with resulting modulation of gene expression. In patients with MPN, the aberrant myeloproliferation is the result of dysregulated JAK2-STAT signaling as well as excess production of inflammatory cytokines associated with this abnormal signaling. These cytokines contribute to the symptoms often reported by patients with MF. JAK2 mutations such as JAK2 V617F are seen in approximately 60% of the patients with PMF and ET and 95% of patients with PV. Unlike CML where the BCR-ABL fusion gene triggers the disease, JAK2 mutations are not initiators of the disease and are not specific for MPN. Further, several other genetic events may contribute to the abnormal JAK2-STAT signaling.
The JAK-STAT signaling pathway has been implicated in the pathogenesis of Myelofibrosis. This pathway normally is responsible for passing information from outside the cell through the cell membrane to the DNA in the nucleus, for gene transcription. Janus Kinase (JAK) family of tyrosine kinases are cytoplasmic proteins and include JAK1, JAK2, JAK3 and TYK2. JAK1 helps propagate the signaling of inflammatory cytokines whereas JAK2 is essential for growth and differentiation of hematopoietic stem cells. These tyrosine kinases mediate cell signaling by recruiting STAT’s (Signal Transducer and Activator of Transcription), with resulting modulation of gene expression. In patients with MPN, the aberrant myeloproliferation is the result of dysregulated JAK2-STAT signaling as well as excess production of inflammatory cytokines associated with this abnormal signaling. These cytokines contribute to the symptoms often reported by patients with MF. JAK2 mutations such as JAK2 V617F are seen in approximately 60% of the patients with PMF and ET and 95% of patients with PV. Unlike CML where the BCR-ABL fusion gene triggers the disease, JAK2 mutations are not initiators of the disease and are not specific for MPN. Further, several other genetic events may contribute to the abnormal JAK2-STAT signaling.
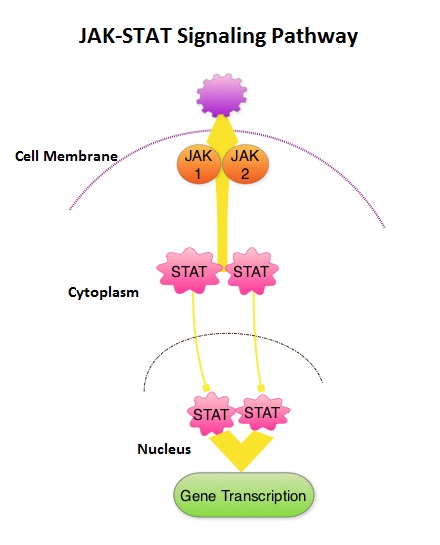 This pathway normally is responsible for passing information from outside the cell through the cell membrane to the DNA in the nucleus for gene transcription. Janus Kinase (JAK) family of tyrosine kinases are cytoplasmic proteins and include JAK1, JAK2, JAK3 and TYK2. JAK1 helps propagate the signaling of inflammatory cytokines whereas JAK2 is essential for growth and differentiation of hematopoietic stem cells. These tyrosine kinases mediate cell signaling by recruiting STAT’s (Signal Transducer and Activator of Transcription), with resulting modulation of gene expression. In patients with MPN, the aberrant myeloproliferation is the result of dysregulated JAK2-STAT signaling as well as excess production of inflammatory cytokines associated with this abnormal signaling. These cytokines contribute to the symptoms often reported by patients with MF. JAK2 mutations such as JAK2 V617F are seen in approximately 60% of the patients with PMF and ET and 95% of patients with PV. Unlike CML where the BCR-ABL fusion gene triggers the disease, JAK2 mutations are not initiators of the disease and are not specific for MPN. Further, several other genetic events may contribute to the abnormal JAK2-STAT signaling. JAKAFI® is a potent JAK1 and JAK2 inhibitor and exerts its mechanism of action by targeting and inhibiting the dysregulated JAK2-STAT signaling pathway. The FDA approval of JAKAFI® for the treatment of Intermediate and high risk Myelofibrosis was based on 2 phase III trials – COMFORT (Controlled Myelofibrosis Study with Oral JAK1/JAK2 Inhibitor Treatment) – I and COMFORT-II studies. In COMFORT-I study, 309 intermediate or high risk patients were randomized to receive either JAKAFI® (N=155) or Placebo (N=154). The primary end point of a 35% or more reduction in spleen size at 24 weeks was noted in 42% of those who received JAKAFI® vs 0.7% in the placebo group (P<0.0001). Most patients in the JAKAFI® group had some reduction in the spleen volume whereas majority of those in the placebo arm had increase in splenomegaly. There was a 46% reduction in the TSS (Total Symptom Score) at week 24 in the JAKAFI® group compared to 5% in the placebo group and majority of patients in the later group had worsening of symptoms (P<0.0001). When JAKAFI® was compared to Best Available Therapy (BAT) in the COMFORT-II study, 28% of the patients in the JAKAFI® group met the primary endpoint of a 35% or more reduction in the spleen volume at 48 weeks compared to none in the BAT group (P<0.0001). Over 55% had a mean decrease in spleen size in the JAKAFI® compared to a 4% mean increase in the BAT group. The 2 year follow up analyses from both these trials showed improved overall survival and a reduction in the risk of death for patients randomized to JAKAFI®, compared to those in the control groups. There was weight gain with alleviation of cachexia and improvements in splenomegaly and symptoms were durable. This benefit was seen in patients regardless of JAK mutations. It remains to be seen if JAKAFI® will benefit patients with Polycythemia Vera and Essential Thrombocythemia. Kantarjian HM, Silver RT, Komrokji RS, et al. Clinical Lymphoma Myeloma and Leukemia 2013; 13:638-645
This pathway normally is responsible for passing information from outside the cell through the cell membrane to the DNA in the nucleus for gene transcription. Janus Kinase (JAK) family of tyrosine kinases are cytoplasmic proteins and include JAK1, JAK2, JAK3 and TYK2. JAK1 helps propagate the signaling of inflammatory cytokines whereas JAK2 is essential for growth and differentiation of hematopoietic stem cells. These tyrosine kinases mediate cell signaling by recruiting STAT’s (Signal Transducer and Activator of Transcription), with resulting modulation of gene expression. In patients with MPN, the aberrant myeloproliferation is the result of dysregulated JAK2-STAT signaling as well as excess production of inflammatory cytokines associated with this abnormal signaling. These cytokines contribute to the symptoms often reported by patients with MF. JAK2 mutations such as JAK2 V617F are seen in approximately 60% of the patients with PMF and ET and 95% of patients with PV. Unlike CML where the BCR-ABL fusion gene triggers the disease, JAK2 mutations are not initiators of the disease and are not specific for MPN. Further, several other genetic events may contribute to the abnormal JAK2-STAT signaling. JAKAFI® is a potent JAK1 and JAK2 inhibitor and exerts its mechanism of action by targeting and inhibiting the dysregulated JAK2-STAT signaling pathway. The FDA approval of JAKAFI® for the treatment of Intermediate and high risk Myelofibrosis was based on 2 phase III trials – COMFORT (Controlled Myelofibrosis Study with Oral JAK1/JAK2 Inhibitor Treatment) – I and COMFORT-II studies. In COMFORT-I study, 309 intermediate or high risk patients were randomized to receive either JAKAFI® (N=155) or Placebo (N=154). The primary end point of a 35% or more reduction in spleen size at 24 weeks was noted in 42% of those who received JAKAFI® vs 0.7% in the placebo group (P<0.0001). Most patients in the JAKAFI® group had some reduction in the spleen volume whereas majority of those in the placebo arm had increase in splenomegaly. There was a 46% reduction in the TSS (Total Symptom Score) at week 24 in the JAKAFI® group compared to 5% in the placebo group and majority of patients in the later group had worsening of symptoms (P<0.0001). When JAKAFI® was compared to Best Available Therapy (BAT) in the COMFORT-II study, 28% of the patients in the JAKAFI® group met the primary endpoint of a 35% or more reduction in the spleen volume at 48 weeks compared to none in the BAT group (P<0.0001). Over 55% had a mean decrease in spleen size in the JAKAFI® compared to a 4% mean increase in the BAT group. The 2 year follow up analyses from both these trials showed improved overall survival and a reduction in the risk of death for patients randomized to JAKAFI®, compared to those in the control groups. There was weight gain with alleviation of cachexia and improvements in splenomegaly and symptoms were durable. This benefit was seen in patients regardless of JAK mutations. It remains to be seen if JAKAFI® will benefit patients with Polycythemia Vera and Essential Thrombocythemia. Kantarjian HM, Silver RT, Komrokji RS, et al. Clinical Lymphoma Myeloma and Leukemia 2013; 13:638-645