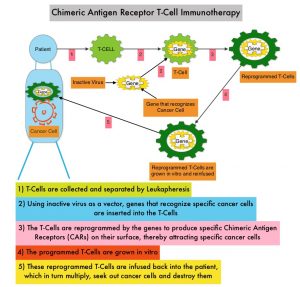
SUMMARY: Multiple Myeloma is a clonal disorder of plasma cells in the bone marrow and the American Cancer Society estimates that in the United States, 34,470 new cases will be diagnosed in 2022 and 12,640 patients will die of the disease. Multiple Myeloma is a disease of the elderly, with a median age at diagnosis of 69 years and characterized by intrinsic clonal heterogeneity. Almost all patients eventually will relapse, and patients with a high-risk cytogenetic profile, extramedullary disease or refractory disease have the worst outcomes. The introduction of Proteasome Inhibitors, immunomodulatory agents and CD 38 targeted therapies has resulted in higher Response Rates, as well as longer Progression Free Survival (PFS) and Overall Survival (OS), with the median survival for patients with myeloma approaching 10 years or more. Nonetheless, Multiple Myeloma (MM) in 2022 remains an incurable disease.
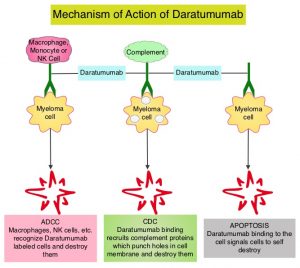
CD38 is a transmembrane glycoprotein, abundantly expressed on malignant plasma cells, and with low levels of expression on normal lymphoid and myeloid cells. DARZALEX® (Daratumumab) is a human IgG1 antibody that targets CD38 and was approved for use in combination with POMALYST® (Pomalidomide) and Dexamethasone in 2017, for the treatment of patients with multiple myeloma, who have received at least two prior therapies including REVLIMID® (Lenalidomide) and a Proteasome Inhibitor. DARZALEX® exerts its cytotoxic effect on myeloma cells by multiple mechanisms, including Antibody Dependent Cellular Cytotoxicity (ADCC), Complement Mediated Cytotoxicity and direct apoptosis. Additionally, DARZALEX® may have a role in immunomodulation by depleting CD38-positive regulator Immune suppressor cells, and thereby expanding T cells, in patients responding to therapy.
SARCLISA® (Isatuximab) is a CD38-targeting monoclonal antibody, similar to DARZALEX®, but unlike DARZALEX®, is not associated with complement activation, and can therefore be more readily given to patients with asthma or Chronic Obstructive Pulmonary Disease. Further, SARCLISA® targets a specific epitope on the CD38 receptor, and this distinction from DARZALEX® allows use of SARCLISA® in cases when DARZALEX® fails. Additionally, SARCLISA® infusions are less cumbersome.
The FDA approval of SARCLISA® in 2020 was based on ICARIA-MM trial, which is an open-label, randomized, multicentre Phase III study in which 307 adult patients with Relapsed and Refractory multiple myeloma who had received at least two previous lines of treatment, including REVLIMID® and a Proteasome Inhibitor were eligible. Patients were excluded if they were refractory to previous treatment with an anti-CD38 monoclonal antibody. Patients were randomly assigned 1:1 to receive either SARCLISA® along with POMALYST® and low-dose Dexamethasone (N =154) or POMALYST® and low-dose Dexamethasone alone (N = 153). Treatment consisted of 28-day cycles of SARCLISA® 10 mg/kg given IV on days 1, 8, 15, and 22 in the first cycle and days 1 and 15 in subsequent cycles. Both groups received POMALYST® 4 mg orally on days 1 to 21 of each cycle and Dexamethasone 40 mg (20 mg for patients aged 75 years or older) orally or IV on days 1, 8, 15, and 22 of each cycle. Treatment was continued until disease progression or unacceptable toxicity. The Primary endpoint was Progression Free Survival (PFS), determined by an Independent Response Committee, and assessed in the intent-to-treat population.
At a median follow up of 11.6 months, the median PFS was 11.5 months in the SARCLISA® group versus 6.5 months in the control group (HR= 0.596; P=0.001). This PFS improvement represented an approximately 40% reduction in the risk of disease progression or death in the SARCLISA® group and was noted in all poor prognostic patient subgroups, including patients who were refractory to REVLIMID®, a Proteasome Inhibitor or both.
The researchers in this publication reported prespecified updated Overall Survival analysis, at 24 months after the primary analysis (second interim analysis). The median follow-up at data cutoff was 35.3 months. The median Overall Survival was 24.6 months in the SARCLISA® group and 17.7 months in the control group (HR=0.76; P=0.028, not crossing prespecified stopping boundary). Final Overall Survival analysis follow up is ongoing. Updated median PFS was 11.1 months in the SARCLISA® group versus 5.9 months in the control group (HR= 0.60; P<0.0001).
Approximately 60% of patients in the SARCLISA® group and 72% in the control group received subsequent therapy after disease progression. Median time to next treatment was longer in the SARCLISA® group (P<0.0001). Among these patients, 24% received DARZALEX® as subsequent therapy versus 58% in the control group. In a post hoc analysis, median overall survival was 19.9 months among control group patients who received DARZALEX® and 17.4 months among those who did not. Overall, median PFS on subsequent therapy or death was 17.5 months in the SARCLISA® group versus vs 12.9 months in the control group (HR=0.76; P=0.02).
The most common Grade 3 or worse treatment-related adverse events in the SARCLISA® group versus the control group were neutropenia (50% versus 35%), pneumonia (23% versus 21%), and thrombocytopenia (13% versus 12%). No new safety concerns were identified with SARCLISA® plus POMALYST® and Dexamethasone, with longer follow up.
The authors concluded that the addition of SARCLISA® to POMALYST® and Dexamethasone resulted in an approximately 7 month difference in median Overall Survival compared with POMALYST® and Dexamethasone, and is a new standard of care for REVLIMID® and Proteasome Inhibitor-refractory or relapsed multiple myeloma.
Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): follow-up analysis of a randomised, phase 3 study. Richardson PG, Perrot A, San-Miguel J, et al. Lancet Oncol. 2022;23(3):416-427. doi:10.1016/S1470-2045(22)00019-5