The FDA on December 19, 2018 granted accelerated approval to KEYTRUDA® for adult and pediatric patients with recurrent locally advanced or metastatic Merkel Cell Carcinoma (MCC). KEYTRUDA® is a product of Merck & Co. Inc.
Tag: Merkel Cell Carcinoma
BAVENCIO® – First FDA Approved Agent for Merkel Cell Carcinoma
SUMMARY: The FDA on March 23, 2017, granted accelerated approval to BAVENCIO® (Avelumab) for the treatment of patients 12 years and older with metastatic Merkel Cell Carcinoma (MCC). It is estimated that about 1500 cases of MCC are diagnosed in the United States each year and the life expectancy for metastatic Merkel Cell Carcinoma is less than 1 year and is associated mortality three times that of Malignant Melanoma (46% vs. 15% respectively). Merkel Cell Carcinoma, also described as Trabecular tumor of the skin, is rare but aggressive form neuroendocrine skin cancer and is much more common in elderly Caucasians. The rapid rise in the incidence of MCC over the past several years has been attributed to increased life expectancy, more sun exposure and weakened immune systems. Approximately 80% of Merkel Cell Carcinoma tumors have been found to be infected with Merkel Cell PolyomaVirus (MCPyV) and the natural history of the MCC has been linked to virus-specific humoral and cellular immune responses. MCC tumors are able to evade the immune system in spite of persistent expression of immunogenic viral proteins. It has been postulated that a high mutation burden associated with Merkel Cell Carcinomas leads to many new antigens being presented to the immune system. Tumor cells as well as tumor-infiltrating immune cells express PD-L1 (Programmed cell Death Ligands), which can contribute to inhibition of antitumor immune response in the tumor microenvironment. The immune system is harnessed and Cytotoxic T-cell activity is suppressed by the binding of PD-L1 to PD-1(Programmed cell Death 1) and B7.1 receptors found on T cells. Merkel Cell Carcinoma is associated with increased PD-L1 expression.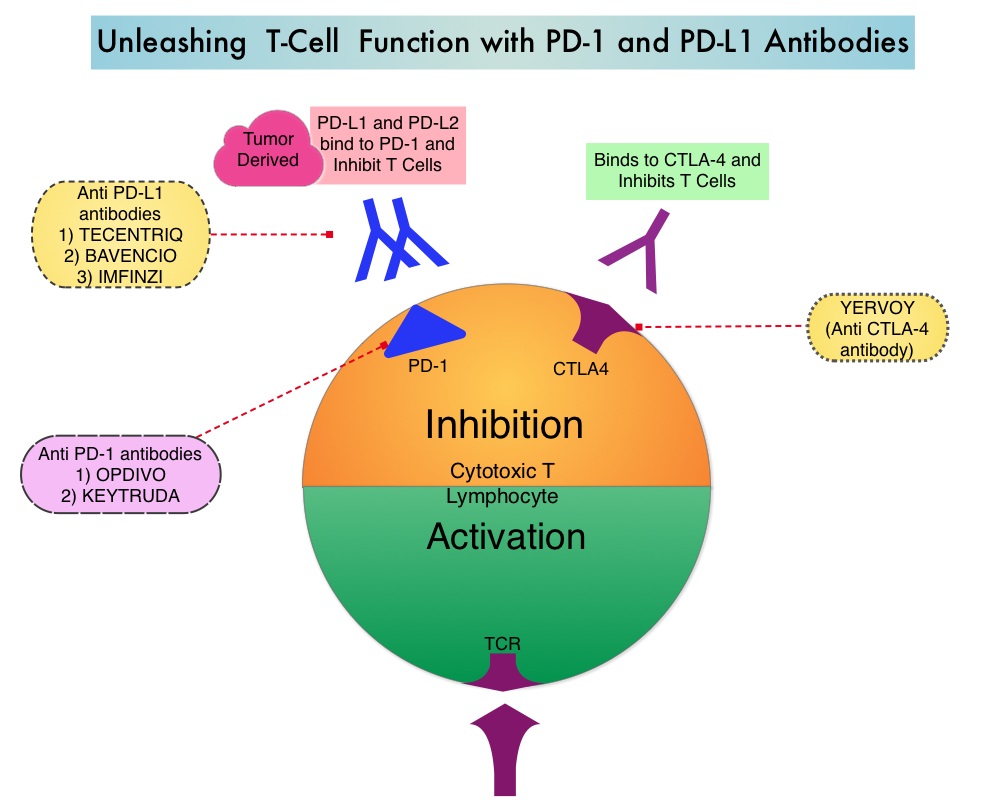
BAVENCIO® is a human, immunoglobulin G1 lambda, PD-L1 targeted monoclonal antibody that binds to PD-L1 and blocks the interaction between PD-L1 and its receptors PD-1. This in turn negates the inhibitory effects of PD-L1 on the immune response by unleashing the immune system and restoring antitumor immune responses. In addition, BAVENCIO® induces Antibody Dependent Cell-mediated Cytotoxicity (ADCC). The approval of BAVENCIO® for Merkel Cell Carcinoma was based on the phase II, prospective, open-label, international JAVELIN trial in which 88 patients with Stage IV Merkel Cell Carcinoma received BAVENCIO® 10 mg/kg IV infusion over 60 minutes, every 2 weeks, until disease progression or unacceptable toxicity. Enrolled patients had at least one prior chemotherapy regimen for metastatic disease. Over 50% of the patients had visceral metastases, two thirds of the patients had tumors with PD-L1 expression of 1% or more, by ImmunoHistoChemistry assay and 52% of the evaluable patients tested positive for Merkel cell Polyomavirus. However, patient selection in this study was not based on the level of PD-L1 expression or Polyomavirus status. The median age was 73 years. The Primary endpoint was Objective Response Rate (ORR). Secondary endpoints included Duration of Response and Progression Free Survival (PFS).
At a median follow up of 16 months, the Objective Response Rate at 1 year was 33% with a Complete Response Rate of 11%. The median time to response was 6 weeks. The 6-month durable response rate was 30.6% and the median Duration of Response had not yet been reached. These responses were noted irrespective of PD-L1 tumor cell expression or presence of Merkel cell Polyomavirus. The estimated one year PFS was 30% and one year Overall Survival was 52%. The most common adverse reactions were rash, fatigue, nausea, diarrhea, decreased appetite, musculoskeletal pain, infusion-related reaction and peripheral edema.
The authors concluded that BAVENCIO® showed durable antitumor activity with a manageable safety profile, in patients with metastatic Merkel Cell Carcinoma who had progressed on chemotherapy and is an important new treatment option for this patient population. BAVENCIO® is the very first drug approved by the FDA for Merkel Cell Carcinoma. Studies are also underway with KEYTRUDA® (Pembrolizumab), a PD-1 inhibitor, in this patient group, with promising outcomes thus far. Durable responses to avelumab (anti-PD-L1) in patients with Merkel cell carcinoma progressed after chemotherapy: 1-year efficacy update. Kaufman HL, Russell JS, Hamid O, et al. 2017 AACR Annual Meeting. Abstract CT079. Presented April 3, 2017.
FDA Approves BAVENCIO® for Merkel Cell Carcinoma
SUMMARY: The FDA on March 23, 2017, granted accelerated approval to BAVENCIO® (Avelumab) for the treatment of patients 12 years and older with metastatic Merkel Cell Carcinoma (MCC). Even though skin cancer is by far the most common type of cancer in the US, Merkel Cell Carcinoma (MCC) is not common. The American Cancer Society estimates that about 1,500 cases of MCC are diagnosed in the United States each year and over 90% of those diagnosed with MCC are older than 50yrs of age. The incidence of MCC in the US has tripled between 1986 and 2001. Merkel Cell Carcinoma, also known as Trabecular Cancer of the Skin, is much more common in Caucasians than in people of other races, and occurs at increased frequency in individuals who are immunodeficient including patients who are transplant recipients. Other risk factors include exposure to ultraviolet light.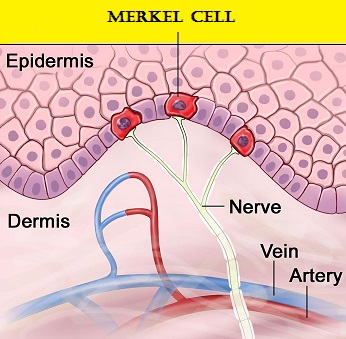
Merkel cells are often found at the base of the epidermis and are responsible for fine touch and pressure sensation. Merkel Cell Carcinoma (MCC) is a very aggressive neuroendocrine carcinoma of the skin. Merkel Cell PolyomaVirus (MCPyV) has been identified in over 80% of Merkel Cell tumors and a majority of MCCs contain clonally integrated viral DNA and express viral T antigen transcripts and protein. The presence of the virus in tumor cells can be confirmed by FISH analysis. This along with ultraviolet radiation-induced mutations provides the rationale for the treatment of MCC, with antibodies that target the PD-L1/PD-1 pathway. BAVENCIO® is a human IgG1 lambda monoclonal antibody, directed against Programmed cell Death Ligand1 (PD-L1) and blocks the interaction of PD-L1 with PD-1. By inhibiting checkpoint proteins and their ligands, T cells are unleashed, resulting in T cell proliferation, activation and a therapeutic response. BAVENCIO® is the first FDA-approved product to treat Merkel Cell Carcinoma.
The approval of BAVENCIO® was based on data from the JAVELIN study which is a multicentre, international, prospective, open-label, phase II trial. This study enrolled 88 previously treated patients with metastatic Merkel Cell Carcinoma. The median age was 72 years. Visceral disease was present in over 50% of patients. Overall, 66% of patients were PD-L1-positive and 52% were positive for the Merkel Cell PolyomaVirus (MCPyV). BAVENCIO® was given intravenously at 10 mg/kg IV every 2 weeks. The Primary endpoint was Objective Response Rate (Complete Response or Partial Response), assessed by an Independent Review Committee. Safety and clinical activity were assessed in all patients who received at least one dose of study drug.
The Objective Response Rate was 33% with 11% Complete Response and 22% Partial Response. Among the responding patients, the response duration ranged from 3 months to 23 months with 86% of responses durable for 6 months or longer. Responses were ongoing in 82% of the patients at the time of analysis. Responses were observed in patients regardless of PD-L1 tumor expression or presence of Merkel Cell PolyomaVirus. The most common adverse reactions were fatigue, musculoskeletal pain, diarrhea, nausea, infusion-related reaction, rash, decreased appetite, and peripheral edema.
The authors concluded that BAVENCIO® is associated with durable response rates and represents a new therapeutic option for advanced Merkel Cell Carcinoma. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Kaufman H, Russell JS, Hamid O, et al. Lancet Oncol. 2016;17:1374-1385
BAVENCIO® (Avelumab)
The FDA on March 23, 2017 granted accelerated approval to BAVENCIO® for the treatment of patients 12 years and older with metastatic Merkel Cell Carcinoma (MCC). BAVENCIO® is a programmed death-ligand 1 (PD-L1) blocking human IgG1 lambda monoclonal antibody. This is the first FDA-approved product to treat this type of cancer. BAVENCIO® is a marketed by EMD Serono, Inc.
