SUMMARY: The FDA on March 14, 2017 granted an accelerated approval to KEYTRUDA® (Pembrolizumab) for the treatment of adult and pediatric patients with classical Hodgkin Lymphoma (cHL) who are Refractory, or have Relapsed, after 3 or more lines of therapy. This approved indication is based on tumor response rate and durability of response. The American Cancer Society estimates that in the United States for 2017, about 8,260 new cases of Hodgkin lymphoma will be diagnosed and about 1,070 patients will die of the disease. Hodgkin lymphoma is classified into two main groups – Classical Hodgkin lymphomas and Nodular Lymphocyte Predominant type, by the World Health Organization. The Classical Hodgkin lymphomas include Nodular sclerosing, Mixed cellularity, Lymphocyte rich, Lymphocyte depleted subtypes and accounts for approximately 10% of all malignant lymphomas. Nodular sclerosis Hodgkin lymphoma histology, accounts for approximately 80% of Hodgkin lymphoma cases in older children and adolescents in the United States. Classical Hodgkin Lymphoma is a malignancy of primarily B lymphocytes and is characterized by the presence of large mononucleated Hodgkin and giant multinucleated Reed-Sternberg (RS) cells collectively known as Hodgkin and Reed-Sternberg cells (HRS). The HRS cells in turn recruit an abundance of ineffective inflammatory cells and infiltrates of immune cells. Preclinical studies suggest that HRS cells evade immune detection by exploiting the pathways associated with immune checkpoint, Programmed Death-1 (PD-1) and its ligands PD-L. Classical Hodgkin Lymphoma is an excellent example of how the tumor microenvironment influences cancer cells to proliferate and survive.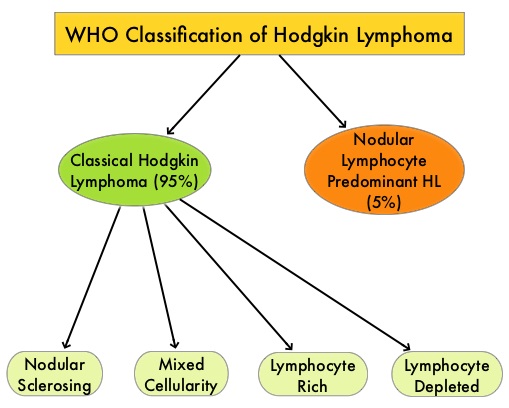
The most common genetic abnormality in Nodular sclerosis subtype of Hodgkin lymphoma is the selective amplification of genes on the short arm of chromosome 9 (9p24.1) which includes JAK-2 with resulting increased expression of PD-1 ligands such as PDL1 and PDL2 on HRS cells, as well as increased JAK-STAT activity, essential for the proliferation and survival of Hodgkin Reed-Sternberg (HRS) cells. Infection with Epstein–Barr virus (EBV) similarly can increase the expression of PDL1 and PDL2 in EBV-positive Hodgkin’s lymphomas. It would therefore seem logical to block or inhibit immune check point PD-1 rather than both its ligands, PDL1 and PDL2. Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Under normal circumstances, Immune checkpoints or gate keepers inhibit intense immune responses by switching off the T cells of the immune system. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies have been developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152), PD-1(Programmed cell Death 1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response. KEYTRUDA® is a fully humanized, Immunoglobulin G4, anti-PD-1, monoclonal antibody, that binds to the PD-1 receptor and blocks its interaction with ligands PD-L1 and PD-L2, thereby undoing PD-1 pathway-mediated inhibition of the immune response and unleashing the tumor-specific effector T cells.
The approval of KEYTRUDA® was based on data from the non-randomized, open-label KEYNOTE-087 trial, which included 210 adult patients with Relapsed/Refractory classical Hodgkin Lymphoma. Approximately 58% of patients were refractory to their last prior therapy, including 35% with primary refractory disease and 14% whose disease was chemo-refractory to all prior treatment regimens. Patients received a median number of four prior therapies and prior therapies included Autologous Hematopoietic Stem Cell Transplantation (61%), ADCETRIS® -Brentuximab vedotin (83%), and radiation therapy (36%). The median age was 35 years and 9% of patients were older than 65 years. KEYTRUDA® was administered at 200 mg IV every 3 weeks until disease progression, unacceptable toxicity, or for up to 2 years, in patients who did not have disease progression. Patients were assessed every 12 weeks to determine their disease status. The Primary end point was Overall Response Rate (ORR). Secondary end points included Complete Response Rate (CRR), Duration of Response, Progression Free Survival, and Overall Survival. The median follow-up was 9.4 months.
It was noted that the Overall Response Rate was 69% and this included Complete Responses in 22% of patients and Partial Responses in 47% of patients. The median Duration of Response was 11.1 months. The most common adverse events were fatigue, pyrexia, cough, musculoskeletal pain, diarrhea, and skin rash. Serious adverse reactions occurred in 16% of patients and the most common grade 3/4 treatment-related adverse events were neutropenia, thrombocytopenia and diarrhea. Adverse reactions led to treatment discontinuation in 5% of the patients.
The authors concluded that PD-1 blockade with KEYTRUDA® has significant clinical activity in subsets of heavily pretreated patients with classical Hodgkin Lymphoma, with high Overall Response Rates and Duration of Response. This important study gives a fighting chance for these generally young patients with poor prognosis. Pembrolizumab in Relapsed/Refractory Classical Hodgkin Lymphoma: Primary End Point Analysis of the Phase 2 Keynote-087 Study. Moskowitz CH, Zinzani PL, Fanale MA, et al. Presented at the ASH 58th Annual Meeting & Exposition, San Diego, CA. December 3-6, 2016. Abstract #1107


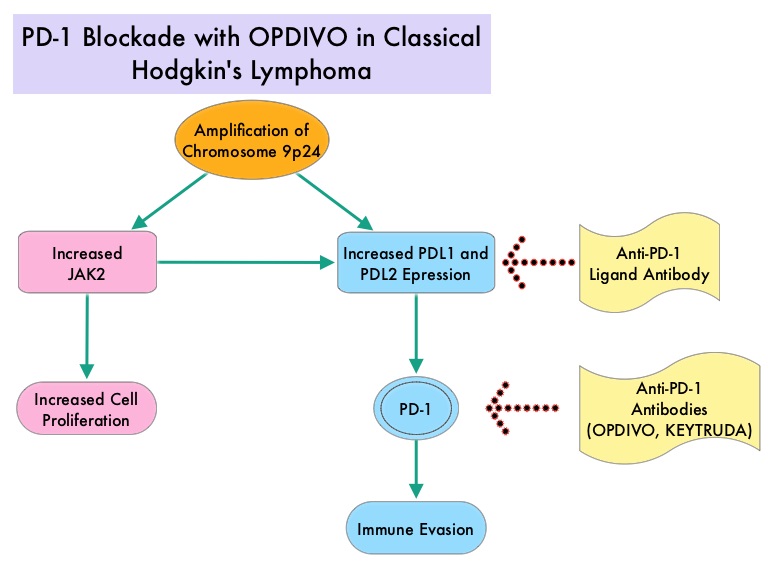 Classical Hodgkin Lymphoma is a malignancy of primarily B lymphocytes and is characterized by the presence of large mononucleated Hodgkin (H) and giant multinucleated Reed-Sternberg (RS) cells collectively known as Hodgkin and Reed-Sternberg cells (HRS). The HRS cells in turn recruit an abundance of ineffective inflammatory cells and infiltrates of immune cells. Preclinical studies suggest that HRS cells evade immune detection by exploiting the pathways associated with immune checkpoint, Programmed Death-1 (PD-1) and its ligands PD-Ls. The most common genetic abnormality in Nodular sclerosis subtype of Hodgkin lymphoma is the selective amplification of genes on the short arm of chromosome 9 (9p24.1) which includes JAK-2 with resulting increased expression of PD-1 ligands such as PDL1 and PDL2 on HRS cells as well as increased JAK-STAT activity, essential for the proliferation and survival of Hodgkin Reed-Sternberg (HRS) cells. Infection with Epstein–Barr virus (EBV) similarly can increase the expression of PDL1 and PDL2 in EBV-positive Hodgkin's lymphomas. It would therefore seem logical to block/inhibit immune check point PD-1 rather than both its ligands, PDL1 and PDL2. Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent related to their ability to escape immune surveillance by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152), PD-1(Programmed cell Death 1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response.
Classical Hodgkin Lymphoma is a malignancy of primarily B lymphocytes and is characterized by the presence of large mononucleated Hodgkin (H) and giant multinucleated Reed-Sternberg (RS) cells collectively known as Hodgkin and Reed-Sternberg cells (HRS). The HRS cells in turn recruit an abundance of ineffective inflammatory cells and infiltrates of immune cells. Preclinical studies suggest that HRS cells evade immune detection by exploiting the pathways associated with immune checkpoint, Programmed Death-1 (PD-1) and its ligands PD-Ls. The most common genetic abnormality in Nodular sclerosis subtype of Hodgkin lymphoma is the selective amplification of genes on the short arm of chromosome 9 (9p24.1) which includes JAK-2 with resulting increased expression of PD-1 ligands such as PDL1 and PDL2 on HRS cells as well as increased JAK-STAT activity, essential for the proliferation and survival of Hodgkin Reed-Sternberg (HRS) cells. Infection with Epstein–Barr virus (EBV) similarly can increase the expression of PDL1 and PDL2 in EBV-positive Hodgkin's lymphomas. It would therefore seem logical to block/inhibit immune check point PD-1 rather than both its ligands, PDL1 and PDL2. Immune checkpoints are cell surface inhibitory proteins/receptors that are expressed on activated T cells. They harness the immune system and prevent uncontrolled immune reactions. Survival of cancer cells in the human body may be to a significant extent related to their ability to escape immune surveillance by inhibiting T lymphocyte activation. The T cells of the immune system therefore play a very important role in modulating the immune system. Under normal circumstances, inhibition of an intense immune response and switching off the T cells of the immune system, is an evolutionary mechanism and is accomplished by Immune checkpoints or gate keepers. With the recognition of Immune checkpoint proteins and their role in suppressing antitumor immunity, antibodies are being developed that target the membrane bound inhibitory Immune checkpoint proteins/receptors such as CTLA-4 (Cytotoxic T-Lymphocyte Antigen 4, also known as CD152), PD-1(Programmed cell Death 1), etc. By doing so, one would expect to unleash the T cells, resulting in T cell proliferation, activation and a therapeutic response.  The authors in this study enrolled 23 patients with relapsed or refractory Hodgkin's lymphoma of whom 87% had received three or more prior therapies. Seventy eight percent (78%) of the patients had previously received ADCETRIS® (Brentuximab) and 78% had undergone autologous stem-cell transplantation. All patients with the exception of one patient had the nodular-sclerosis subtype of Hodgkin's lymphoma. The median age was 35 years. Patients received OPDIVO® (Nivolumab) at a dose of 3 mg/kg every 2 weeks and treatment was continued until patients had a complete response, tumor progression, or severe toxicities. OPDIVO® is an immune checkpoint PD-1 (Programmed cell Death 1) targeted, fully human, immunoglobulin G4 monoclonal antibody. The median number of OPDIVO® doses that patients received was 16 and the median duration of treatment was 36 weeks. The authors noted an Objective Response Rate of 87% with a complete response of 17%, and a partial response of 70%, in this heavily pretreated group of patients. Stable disease was noted in 13% of the patients. The Progression Free Survival at 24 weeks was 86%.Most of the adverse events were grade 1 and grade 2. Analyses of pretreatment tumor specimens revealed increased expression of PDL1 and PDL2. Reed–Sternberg cells showed nuclear positivity of phosphorylated STAT3, suggestive of active JAK-STAT signaling. The authors concluded that OPDIVO® is highly active with favorable toxicities in heavily pretreated relapsed or refractory Hodgkin's lymphoma “highlighting the genetically defined sensitivity to PD-1 blockade”. PD-1 Blockade with Nivolumab in Relapsed or Refractory Hodgkin's Lymphoma. Ansell SM, Lesokhin AM, Borrello I, et al. N Engl J Med 2015; 372:311-319
The authors in this study enrolled 23 patients with relapsed or refractory Hodgkin's lymphoma of whom 87% had received three or more prior therapies. Seventy eight percent (78%) of the patients had previously received ADCETRIS® (Brentuximab) and 78% had undergone autologous stem-cell transplantation. All patients with the exception of one patient had the nodular-sclerosis subtype of Hodgkin's lymphoma. The median age was 35 years. Patients received OPDIVO® (Nivolumab) at a dose of 3 mg/kg every 2 weeks and treatment was continued until patients had a complete response, tumor progression, or severe toxicities. OPDIVO® is an immune checkpoint PD-1 (Programmed cell Death 1) targeted, fully human, immunoglobulin G4 monoclonal antibody. The median number of OPDIVO® doses that patients received was 16 and the median duration of treatment was 36 weeks. The authors noted an Objective Response Rate of 87% with a complete response of 17%, and a partial response of 70%, in this heavily pretreated group of patients. Stable disease was noted in 13% of the patients. The Progression Free Survival at 24 weeks was 86%.Most of the adverse events were grade 1 and grade 2. Analyses of pretreatment tumor specimens revealed increased expression of PDL1 and PDL2. Reed–Sternberg cells showed nuclear positivity of phosphorylated STAT3, suggestive of active JAK-STAT signaling. The authors concluded that OPDIVO® is highly active with favorable toxicities in heavily pretreated relapsed or refractory Hodgkin's lymphoma “highlighting the genetically defined sensitivity to PD-1 blockade”. PD-1 Blockade with Nivolumab in Relapsed or Refractory Hodgkin's Lymphoma. Ansell SM, Lesokhin AM, Borrello I, et al. N Engl J Med 2015; 372:311-319