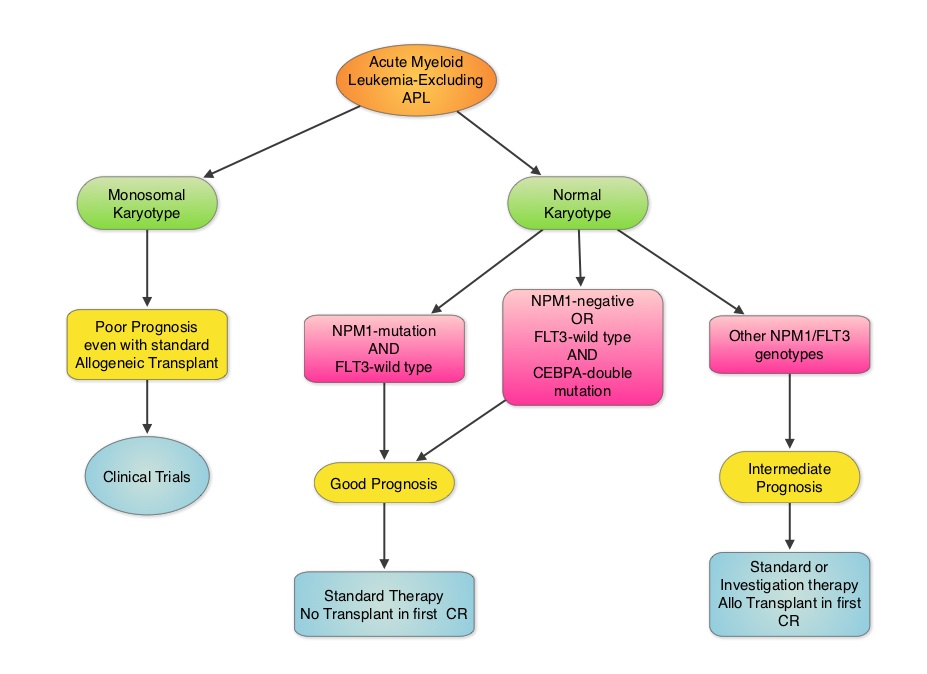
SUMMARY: Cytogenetic analysis has been part of routine evaluation when caring for patients with AML. By predicting resistance to therapy, cytogenetics stratify patients based on risk and helps manage them accordingly. Even though normal karyotype is the most common cytogenetic finding, approximately 10%-15% of AML patients have a monosomal karyotype (presence of at least 2 autosomal monosomies or a single autosomal monosomy in combination with at least one structural abnormality). These patients have a poor prognosis and alterations in the TP53 gene has been implicated in majority of these patients. AML patients with a normal karyotype should be tested for NPM1 (Nucleophosmin), FLT3 (Fms-related tyrosine kinase 3) and CEBPA (CCAAT/Enhancer Binding Protein Alpha) mutations, in addition to cytogenetics, as this may have therapeutic implications. CEBPA is a transcription factor and plays an important role in myeloid differentiation. Mutations in the CEBPA gene have been described in approximately 10% of patients with AML. Patients can have one or two mutations in this gene. It appears that favorable outcomes may be limited to those patients who have double CEBPA mutations rather than those with single CEBPA mutations. AML patients without FLT3 mutations or NPM1 mutation with CEBPA-double mutations have a favorable outcome. In this retrospective review, the authors analyzed the clinical impact of NPM1 and FLT3 mutations in AML patients, 65 years of age or older, treated with cytotoxic chemotherapy. A total of 557 patients were retrospectively reviewed. They noted that the outcomes were significantly better amongst patients with NPM1-mut/FLT3-wild type genotype compared to any other NPM1/FLT3 genotypes. The median survival was 21.5 months vs. 9.0 months and estimated 2-year survival rates were 51% vs. 38%, respectively (P = .003). The authors concluded that elderly AML patients with NPM1-mut/FLT3-wild type genotype have significantly improved outcomes when treated with cytotoxic chemotherapy. This subset of patients have a good prognosis with outcomes similar to those with favorable cytogenetics such as Inversion 16 and t(8:21). The discovery of molecular mutations is providing valuable information to facilitate risk-adapted therapy. Daver N, Liu Dumlao T, Ravandi F, et al. Clinical Lymphoma Myeloma and Leukemia 2013;13:435-440