SUMMARY: The American Cancer Society estimates that in 2014, 18,860 new cases of Acute Myeloid Leukemia (AML) will be diagnosed in the United States and 10,460 patients will die of the disease. Acute Myeloid Leukemia in general is a disease of the elderly and the average age of a patient with AML is about 66 years. AML can be considered as a group of heterogeneous diseases with different clinical behavior and outcomes. Cytogenetic analysis has been part of routine evaluation when caring for patients with AML. By predicting resistance to therapy, tumor cytogenetics will stratify patients, based on risk and help manage them accordingly. Even though cytotoxic chemotherapy may lead to long term remission and cure in a minority of patients with favorable cytogenetics, patients with high risk features such as unfavorable cytogenetics, molecular abnormalities, prior myelodysplasia and advanced age, have poor outcomes with conventional chemotherapy.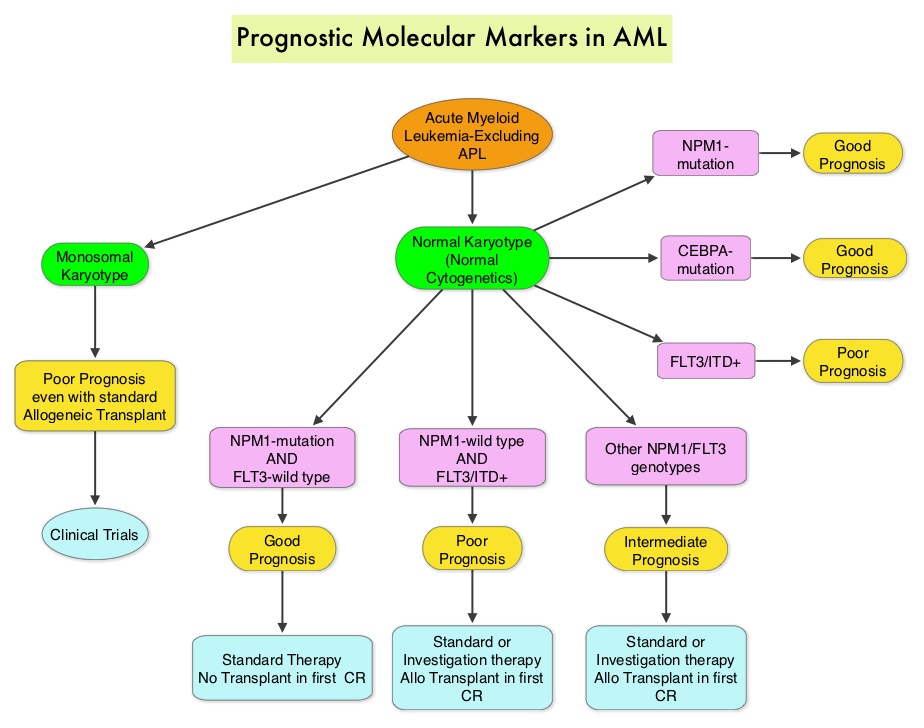 The Fms-Like Tyrosine kinase 3 (FLT3) is a receptor tyrosine kinase in the PDGF family of growth factor receptors located on the cell surface (transmembrane) and plays an important role in both normal and malignant hematopoiesis by activating key signaling pathways. Activating mutations in the FLT3 receptor is the most common genetic abnormality in AML and is detected in approximately 30% of the patients. The most common FLT3 mutation is the FLT3-ITD (Internal Tandem Duplication) mutation caused by tandem duplication within the coding region of the gene. The presence of FLT3-ITD mutations can negate the benefit of any other favorable molecular and cytogenetic features. Patients with FLT3-ITD mutations are predicted to have poor outcomes with shorter remission duration and significantly decreased leukemia free and overall survival. These mutations are detected using Polymerase Chain Reaction (PCR) based molecular diagnostic DNA testing. The authors in this meta-analysis examined the prognostic significance of three mutations frequently noted in patients with cytogenetically normal Acute Myeloid Leukemia. These mutations included FLT3-ITD, mutated NPM1 (Nucleophosmin) and mutations of the CCAAT enhancer-binding protein alpha (CEBPA) gene. This systematic review and meta-analysis included 1942 patients from multiple electronic databases from 2000 to March 2012. It was noted that FLT3-ITD was associated with the worse prognosis, with inferior Overall Survival (OS) and Relapse Free Survival (RFS), whereas mutations in NPM1 and CEBPA genes were associated with a favorable prognosis. The discovery of new molecular mutations in AML patients with normal cytogenetics may help predict outcomes and provide valuable information to facilitate risk-adapted therapy. Port M, Böttcher M, Thol F, et al. Ann Hematol. 2014;93:1279-1286
The Fms-Like Tyrosine kinase 3 (FLT3) is a receptor tyrosine kinase in the PDGF family of growth factor receptors located on the cell surface (transmembrane) and plays an important role in both normal and malignant hematopoiesis by activating key signaling pathways. Activating mutations in the FLT3 receptor is the most common genetic abnormality in AML and is detected in approximately 30% of the patients. The most common FLT3 mutation is the FLT3-ITD (Internal Tandem Duplication) mutation caused by tandem duplication within the coding region of the gene. The presence of FLT3-ITD mutations can negate the benefit of any other favorable molecular and cytogenetic features. Patients with FLT3-ITD mutations are predicted to have poor outcomes with shorter remission duration and significantly decreased leukemia free and overall survival. These mutations are detected using Polymerase Chain Reaction (PCR) based molecular diagnostic DNA testing. The authors in this meta-analysis examined the prognostic significance of three mutations frequently noted in patients with cytogenetically normal Acute Myeloid Leukemia. These mutations included FLT3-ITD, mutated NPM1 (Nucleophosmin) and mutations of the CCAAT enhancer-binding protein alpha (CEBPA) gene. This systematic review and meta-analysis included 1942 patients from multiple electronic databases from 2000 to March 2012. It was noted that FLT3-ITD was associated with the worse prognosis, with inferior Overall Survival (OS) and Relapse Free Survival (RFS), whereas mutations in NPM1 and CEBPA genes were associated with a favorable prognosis. The discovery of new molecular mutations in AML patients with normal cytogenetics may help predict outcomes and provide valuable information to facilitate risk-adapted therapy. Port M, Böttcher M, Thol F, et al. Ann Hematol. 2014;93:1279-1286
Month: December 2014
Olaparib Monotherapy in Patients with Advanced Cancer and a Germline BRCA1/2 Mutation
SUMMARY:The FDA on December 19, 2014 approved LYNPARZA® (Olaparib) as monotherapy for the treatment of patients with deleterious or suspected deleterious germline BRCA mutated (gBRCAm) advanced ovarian cancer who had been treated with three or more prior lines of chemotherapy. It is estimated that in the United States, approximately 22,000 women will be diagnosed with ovarian cancer in 2014 and a little over 14,000 women will die of the disease. DNA can be damaged due to errors during its replication or as a result of environmental exposure to ultraviolet radiation from the sun or other toxins. The tumor suppressor genes such as BRCA1 (Breast Cancer 1) and BRCA2 help repair damaged DNA and thus play an important role in maintaining cellular genetic integrity, failing which these genetic aberrations can result in malignancies. The BRCA1 gene is located on the long (q) arm of chromosome 17 whereas BRCA2 is located on the long arm of chromosome 13. Mutations in BRCA1 and BRCA2 account for about 20 to 25 percent of hereditary breast cancers and about 5 to 10 percent of all breast cancers. They also account 15 percent of ovarian cancers in addition to other cancers such as colon and prostate. These mutations can be inherited from either of the parents and a child has a 50 percent chance of inheriting this mutation and the deleterious effects of the mutations are seen even when an individual’s second copy of the gene is normal.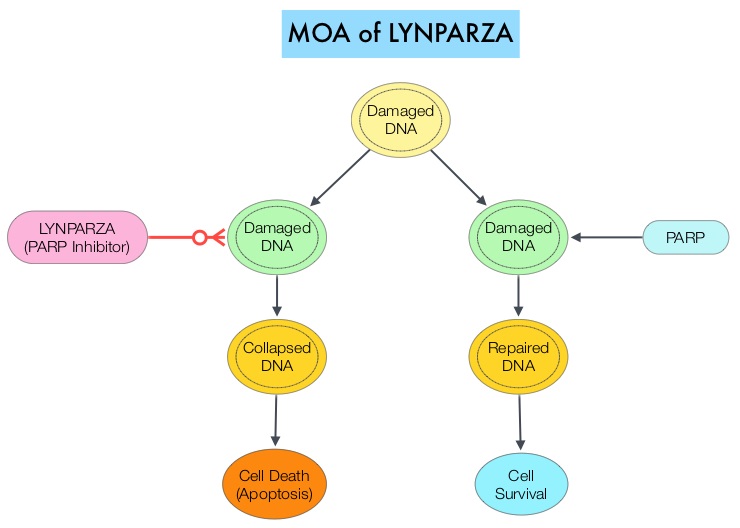 The PARP (Poly ADP Ribose Polymerase) family of enzymes which include PARP1 and PARP2, repair damaged DNA. LYNPARZA® is a PARP enzyme inhibitor that causes cell death in tumors that already have a DNA repair defect, such as those with BRCA1 and BRCA2 mutations. The approval of LYNPARZA® was based on a single arm phase II trial in which 137 platinum resistant ovarian cancer patients with measurable germline BRCA mutations were enrolled. The BRCA mutation status was verified retrospectively in 97% of the patients with available blood samples from the phase II study, using the BRACAnalysis CDx® test. These patients had received three or more lines of prior chemotherapy. Treatment consisted of LYNPARZA® administered orally twice a day and was continued until disease progression or unacceptable toxicity. The primary endpoint was Objective Response Rate (ORR). The Overall Response Rate was 34% and the median response duration was 7.9 months. In a larger cohort of patients reported by the authors (ovarian cancer cohort, N=193) the median Progression Free Survival was 7 months, 55% of patients were progression free at 6 months, the median Overall Survival was 16.6 months and 64.4% of patients were alive at 12 months. The most common adverse reactions associated with LYNPARZA® were anemia, nausea, fatigue (including asthenia), vomiting, diarrhea, dysgeusia, dyspepsia, headache, decreased appetite, nasopharyngitis/pharyngitis/URI, cough, arthralgia/musculoskeletal pain, myalgia, back pain, dermatitis/rash and abdominal pain/discomfort. This ground breaking therapy with LYNPARZA® is first of a new class of drugs, for treating ovarian cancer and along with the BRACAnalysis CDx® companion diagnostic test, is a significant milestone for patients with difficult-to-treat advanced ovarian cancer, with germline BRCA mutations. Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. [published online November 3, 2014]. J Clin Oncol. doi:10.1200/JCO.2014.56.2728.
The PARP (Poly ADP Ribose Polymerase) family of enzymes which include PARP1 and PARP2, repair damaged DNA. LYNPARZA® is a PARP enzyme inhibitor that causes cell death in tumors that already have a DNA repair defect, such as those with BRCA1 and BRCA2 mutations. The approval of LYNPARZA® was based on a single arm phase II trial in which 137 platinum resistant ovarian cancer patients with measurable germline BRCA mutations were enrolled. The BRCA mutation status was verified retrospectively in 97% of the patients with available blood samples from the phase II study, using the BRACAnalysis CDx® test. These patients had received three or more lines of prior chemotherapy. Treatment consisted of LYNPARZA® administered orally twice a day and was continued until disease progression or unacceptable toxicity. The primary endpoint was Objective Response Rate (ORR). The Overall Response Rate was 34% and the median response duration was 7.9 months. In a larger cohort of patients reported by the authors (ovarian cancer cohort, N=193) the median Progression Free Survival was 7 months, 55% of patients were progression free at 6 months, the median Overall Survival was 16.6 months and 64.4% of patients were alive at 12 months. The most common adverse reactions associated with LYNPARZA® were anemia, nausea, fatigue (including asthenia), vomiting, diarrhea, dysgeusia, dyspepsia, headache, decreased appetite, nasopharyngitis/pharyngitis/URI, cough, arthralgia/musculoskeletal pain, myalgia, back pain, dermatitis/rash and abdominal pain/discomfort. This ground breaking therapy with LYNPARZA® is first of a new class of drugs, for treating ovarian cancer and along with the BRACAnalysis CDx® companion diagnostic test, is a significant milestone for patients with difficult-to-treat advanced ovarian cancer, with germline BRCA mutations. Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. [published online November 3, 2014]. J Clin Oncol. doi:10.1200/JCO.2014.56.2728.
Impact on overall survival (OS) with chemohormonal therapy versus hormonal therapy for hormone-sensitive newly metastatic prostate cancer (mPrCa) An ECOG-led phase III randomized trial
SUMMARY: Prostate cancer is the most common cancer in American men, excluding skin cancer and 1 in 7 men will be diagnosed with prostate cancer during their lifetime. It is estimated that in the United States, over 230,000 new cases of prostate cancer will be diagnosed in 2014 and close to 30,000 men will die of the disease.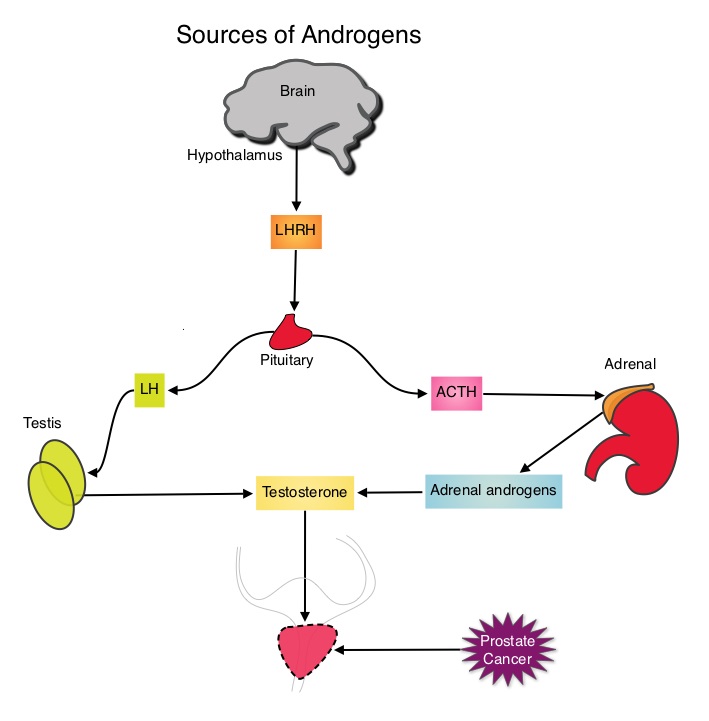 The development and progression of prostate cancer is driven by androgens. Androgen Deprivation Therapy (ADT) has therefore been the cornerstone of treatment of advanced prostate cancer and is the first treatment intervention for hormone sensitive prostate cancer. Chemotherapy is usually considered for patients who progress on hormone therapy and TAXOTERE® (Docetaxel) has been shown to improve Overall Survival (OS) of metastatic prostate cancer patients, who had progressed on androgen deprivation therapy. It is not clear however, whether ADT is more effective with or without TAXOTERE®, when treating patients with metastatic prostate cancer. To address this further, a randomized phase III trial (E3805) was conducted to assess the benefit of upfront treatment with a combination of chemotherapy and hormonal therapy, in patients with metastatic hormone sensitive prostate cancer. Seven hundred and ninety (N=790) patients with newly diagnosed metastatic prostate cancer were randomly assigned to receive either Androgen Deprivation Therapy alone (N=393) or ADT plus TAXOTERE® (N=397). Androgen Deprivation Therapy consisted of either Luteinizing Hormone Releasing Hormone (LHRH) agonist therapy, LHRH antagonist therapy, or surgical castration. Chemotherapy consisted of TAXOTERE®, started within 4 months of starting ADT, dosed at 75 mg/m2 given every 3 weeks for a maximum of six cycles. The median age of patients was 63 years and approximately two-thirds of patients had high-volume disease, with either extensive liver or bone metastases. The primary endpoint of this study was Overall Survival. At a median follow up of 29 months, the median Overall Survival was 42.3 months in the ADT group and 52.7 months in the ADT plus TAXOTERE® group (HR=0.63; P<0.0006). This benefit was even more significant in patients with high volume disease (32.2 vs 49.2 months for ADT and ADT plus TAXOTERE® respectively, HR=0.62; P<0.0012). At 12 months, the proportion of patients with PSA levels less than 0.2 ng/mL was 9.4% in the ADT alone group vs 19.7% in the ADT plus TAXOTERE® group (P < 0.0001). The median time to clinical progression was 19.8 months in the ADT alone group vs 32.7 months in the ADT plus TAXOTERE® group (P < 0.0001). The authors concluded that this is the first study to demonstrate survival benefit in patients with newly diagnosed metastatic prostate cancer. This survival benefit with Androgen Deprivation Therapy and TAXOTERE® is even more so, in patients with high volume disease and should be considered standard treatment for those patients who are fit to receive TAXOTERE® based therapy. Sweeney C, Chen Y, Carducci MA, et al. 2014 ASCO Annual Meeting; LBA2
The development and progression of prostate cancer is driven by androgens. Androgen Deprivation Therapy (ADT) has therefore been the cornerstone of treatment of advanced prostate cancer and is the first treatment intervention for hormone sensitive prostate cancer. Chemotherapy is usually considered for patients who progress on hormone therapy and TAXOTERE® (Docetaxel) has been shown to improve Overall Survival (OS) of metastatic prostate cancer patients, who had progressed on androgen deprivation therapy. It is not clear however, whether ADT is more effective with or without TAXOTERE®, when treating patients with metastatic prostate cancer. To address this further, a randomized phase III trial (E3805) was conducted to assess the benefit of upfront treatment with a combination of chemotherapy and hormonal therapy, in patients with metastatic hormone sensitive prostate cancer. Seven hundred and ninety (N=790) patients with newly diagnosed metastatic prostate cancer were randomly assigned to receive either Androgen Deprivation Therapy alone (N=393) or ADT plus TAXOTERE® (N=397). Androgen Deprivation Therapy consisted of either Luteinizing Hormone Releasing Hormone (LHRH) agonist therapy, LHRH antagonist therapy, or surgical castration. Chemotherapy consisted of TAXOTERE®, started within 4 months of starting ADT, dosed at 75 mg/m2 given every 3 weeks for a maximum of six cycles. The median age of patients was 63 years and approximately two-thirds of patients had high-volume disease, with either extensive liver or bone metastases. The primary endpoint of this study was Overall Survival. At a median follow up of 29 months, the median Overall Survival was 42.3 months in the ADT group and 52.7 months in the ADT plus TAXOTERE® group (HR=0.63; P<0.0006). This benefit was even more significant in patients with high volume disease (32.2 vs 49.2 months for ADT and ADT plus TAXOTERE® respectively, HR=0.62; P<0.0012). At 12 months, the proportion of patients with PSA levels less than 0.2 ng/mL was 9.4% in the ADT alone group vs 19.7% in the ADT plus TAXOTERE® group (P < 0.0001). The median time to clinical progression was 19.8 months in the ADT alone group vs 32.7 months in the ADT plus TAXOTERE® group (P < 0.0001). The authors concluded that this is the first study to demonstrate survival benefit in patients with newly diagnosed metastatic prostate cancer. This survival benefit with Androgen Deprivation Therapy and TAXOTERE® is even more so, in patients with high volume disease and should be considered standard treatment for those patients who are fit to receive TAXOTERE® based therapy. Sweeney C, Chen Y, Carducci MA, et al. 2014 ASCO Annual Meeting; LBA2
Confirmatory open-label, single-arm, multicenter phase 2 study of the BiTE antibody, Blinatumomab in patients (pts) with relapsed/refractory B-precursor acute lymphoblastic leukemia (r/r ALL)
SUMMARY: The FDA on December 3, 2014, granted accelerated approval to BLINCYTO® (Blinatumomab), a bispecific T cell engager (BiTE) antibody, for treatment of Philadelphia chromosome-negative (Ph-) Relapsed or Refractory B- cell precursor Acute Lymphoblastic Leukemia (ALL). BiTE® technology engages the body's immune system to detect and target malignant cells. These modified antibodies are designed to engage two different targets simultaneously, thereby placing the T cells within reach of the targeted cancer cell and facilitating apoptosis of the cancer cell. BiTE® antibodies are currently being investigated to treat a wide variety of malignancies.  BLINCYTO® (Blinatumomab) is an investigational BiTE® antibody designed to direct the patients T cells against CD19, a protein found on the surface of B-cell derived leukemias and lymphomas. The approval was based on a multicenter single-arm phase II trial in which 185 patients with Relapsed or Refractory Philadelphia chromosome negative ALL patients were enrolled. The median age was 39 years, and patients had their 1st relapse and were refractory to post hematopoietic stem cell transplantation less than 12 months before. About a third of the patients had at least 2 salvage therapies. BLINCYTO® was given by continuous IV infusion, 4 weeks on and 2 weeks off for up to 5 cycles and the median number of cycles given were 2. The primary endpoint was complete remission (CR) and response with a reduction in Minimal Residual Disease (MRD) to less than 10-4 or CR with partial hematological recovery (CRh), within the first 2 cycles of treatment. It was noted that 32% of patients attained CR with 2 cycles of treatment with BLINCYTO® and these responses were durable (median 6.7 months). Further, 31% of the patients in this study had a CR with or without complete hematological recovery but with reduction in MRD to less than 10-4. At the time of primary analysis, 80% of responses occurred within cycle 1. Further, the Complete Remissions (CR) and CR with partial hematological recovery (CRh) were seen in all subgroups of patients, although this was more pronounced in those with less than 50% bone marrow blasts. The median Relapse Free Survival and Overall survival were 5.9 months and 6.1 months respectively. The most frequent grade 3 adverse events were febrile neutropenia, neutropenia and anemia, occurring in 26%, 15% and 15% of patients, respectively. The authors concluded that BLINCYTO® has significant single agent antileukemia activity in a difficult-to-treat population with Relapsed and Refractory Acute Lymphoblastic Leukemia. Future studies will hopefully address whether BLINCYTO® can serve as a bridge to transplantation, in patients with Relapsed and Refractory B-cell ALL. Cytokine Release Syndrome can result from the activation of the immune system. The FDA approved BLINCYTO® with a Risk Evaluation and Mitigation Strategy (REMS). Topp MS, Goekbuget N, Stein AS, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 7005)
BLINCYTO® (Blinatumomab) is an investigational BiTE® antibody designed to direct the patients T cells against CD19, a protein found on the surface of B-cell derived leukemias and lymphomas. The approval was based on a multicenter single-arm phase II trial in which 185 patients with Relapsed or Refractory Philadelphia chromosome negative ALL patients were enrolled. The median age was 39 years, and patients had their 1st relapse and were refractory to post hematopoietic stem cell transplantation less than 12 months before. About a third of the patients had at least 2 salvage therapies. BLINCYTO® was given by continuous IV infusion, 4 weeks on and 2 weeks off for up to 5 cycles and the median number of cycles given were 2. The primary endpoint was complete remission (CR) and response with a reduction in Minimal Residual Disease (MRD) to less than 10-4 or CR with partial hematological recovery (CRh), within the first 2 cycles of treatment. It was noted that 32% of patients attained CR with 2 cycles of treatment with BLINCYTO® and these responses were durable (median 6.7 months). Further, 31% of the patients in this study had a CR with or without complete hematological recovery but with reduction in MRD to less than 10-4. At the time of primary analysis, 80% of responses occurred within cycle 1. Further, the Complete Remissions (CR) and CR with partial hematological recovery (CRh) were seen in all subgroups of patients, although this was more pronounced in those with less than 50% bone marrow blasts. The median Relapse Free Survival and Overall survival were 5.9 months and 6.1 months respectively. The most frequent grade 3 adverse events were febrile neutropenia, neutropenia and anemia, occurring in 26%, 15% and 15% of patients, respectively. The authors concluded that BLINCYTO® has significant single agent antileukemia activity in a difficult-to-treat population with Relapsed and Refractory Acute Lymphoblastic Leukemia. Future studies will hopefully address whether BLINCYTO® can serve as a bridge to transplantation, in patients with Relapsed and Refractory B-cell ALL. Cytokine Release Syndrome can result from the activation of the immune system. The FDA approved BLINCYTO® with a Risk Evaluation and Mitigation Strategy (REMS). Topp MS, Goekbuget N, Stein AS, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 7005)
Results of a prospective, randomized, open-label phase 3 study of ruxolitinib (RUX) in polycythemia vera (PV) patients resistant to or intolerant of hydroxyurea (HU) the RESPONSE trial
SUMMARY:The FDA on December 4, 2014 approved JAKAFI® (Ruxolitinib) for the treatment of patients with Polycythemia Vera (P.Vera) who have had an inadequate response to or are intolerant of Hydroxyurea (HU). Polycythemia Vera is a clonal myeloproliferative neoplasm characterized by isolated Erythrocytosis in a majority of the patients, with the remaining demonstrating Leukocytosis and/or Thrombocytosis along with Erythrocytosis. Patients usually present with this disorder in their sixth decade and are often asymptomatic, with the diagnosis made incidentally on routine laboratory evaluation. About 30% of the patients however, may initially present with a thrombotic episode, whereas a small percentage of patients may present with disease related symptoms such as pruritus and fatigue.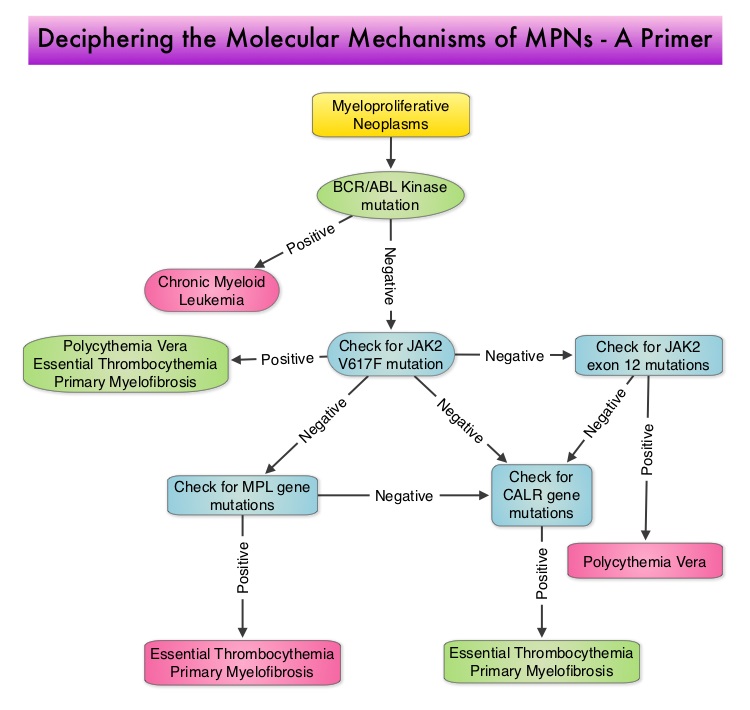 Overactivation of the JAK-STAT signal transduction pathway caused by V617F mutation, has been implicated in majority of the patients with P. Vera. This pathway normally is responsible for passing information from outside the cell through the cell membrane to the DNA in the nucleus, for gene transcription. Janus Kinase (JAK) family of tyrosine kinases are cytoplasmic proteins and include JAK1, JAK2, JAK3 and TYK2. JAK1 helps propagate the signaling of inflammatory cytokines whereas JAK2 is essential for growth and differentiation of hematopoietic stem cells. These tyrosine kinases mediate cell signaling by recruiting STAT’s (Signal Transducer and Activator of Transcription), with resulting modulation of gene expression. In patients with P. Vera, the aberrant myeloproliferation is the result of dysregulated JAK2-STAT signaling as well as excess production of inflammatory cytokines, associated with this abnormal signaling. JAK2 mutations such as JAK2 V617F are seen in approximately 95% of patients with P. Vera. The goals of therapy in P. Vera are to maintain the hematocrit at less than 45% and decrease the risk of thrombosis and bleeding. P. Vera is presently managed with periodic phlebotomies, cytoreductive therapy with oral antimetabolite, Hydroxyurea and antiplatelet agents such as low dose Aspirin. However, a significant number of patients on these therapies become intolerant or resistant to these treatments, leading to an increased risk of progression.
Overactivation of the JAK-STAT signal transduction pathway caused by V617F mutation, has been implicated in majority of the patients with P. Vera. This pathway normally is responsible for passing information from outside the cell through the cell membrane to the DNA in the nucleus, for gene transcription. Janus Kinase (JAK) family of tyrosine kinases are cytoplasmic proteins and include JAK1, JAK2, JAK3 and TYK2. JAK1 helps propagate the signaling of inflammatory cytokines whereas JAK2 is essential for growth and differentiation of hematopoietic stem cells. These tyrosine kinases mediate cell signaling by recruiting STAT’s (Signal Transducer and Activator of Transcription), with resulting modulation of gene expression. In patients with P. Vera, the aberrant myeloproliferation is the result of dysregulated JAK2-STAT signaling as well as excess production of inflammatory cytokines, associated with this abnormal signaling. JAK2 mutations such as JAK2 V617F are seen in approximately 95% of patients with P. Vera. The goals of therapy in P. Vera are to maintain the hematocrit at less than 45% and decrease the risk of thrombosis and bleeding. P. Vera is presently managed with periodic phlebotomies, cytoreductive therapy with oral antimetabolite, Hydroxyurea and antiplatelet agents such as low dose Aspirin. However, a significant number of patients on these therapies become intolerant or resistant to these treatments, leading to an increased risk of progression.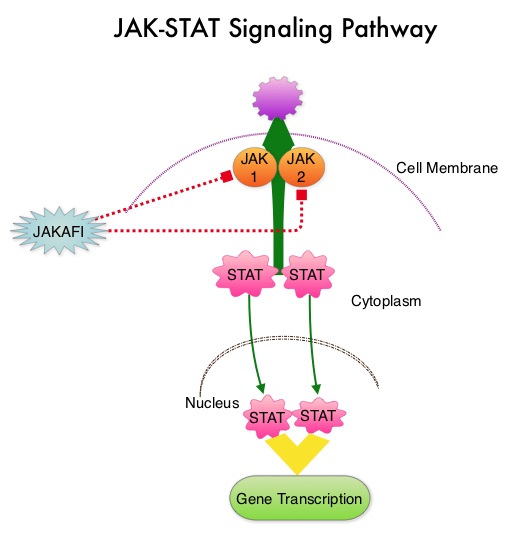 JAKAFI® is a potent JAK1 and JAK2 inhibitor and exerts its mechanism of action by targeting and inhibiting the dysregulated JAK2-STAT signaling pathway. The RESPONSE trial is a phase III prospective randomized study in which patients with P. Vera, who were refractory or intolerant of Hydroxyurea were randomized to receive JAKAFI® 10 mg PO, BID (N=110) or Best Available Therapy (BAT), which consisted of investigator choice of monotherapy or observation only (N=112). Eligible patients were phlebotomy dependent and had splenomegaly (> 450 cubic cm). Patients receiving BAT were allowed to cross over to JAKAFI® group from week 32 onwards. The primary endpoint of this study (composite primary endpoint) was the proportion of patients whose hematocrit was controlled without phlebotomy and whose spleen volume was reduced by 35% or more from baseline, as assessed by MRI imaging at 32 weeks. Secondary endpoints included durable response, Complete Hematological Remission and safety. The primary analysis was conducted when all patients reached week 48 or discontinued therapy. The proportion of patients in the JAKAFI® group who achieved the composite primary endpoint was 21% compared to 1% in the BAT group (P < 0.0001) and 91% in the JAKAFI® group maintained their response at week 48. Seventy seven percent (77%) of the patients in the JAKAFI® group achieved at least one of the two major components of the composite primary endpoint. Put another way, 60% of the patients in the JAKAFI® arm were able to achieve the target hematocrit level in the absence of phlebotomy, compared to 20% in the BAT group. Reduction in the spleen volume by 35% or more was noted in 38% of the patients in the JAKAFI® group compared to 1% in the BAT group. Complete Hematological Remission defined as continuous hematocrit below 45%, as well as normal white blood cells and platelets counts, was achieved in 24% and 9% of patients in JAKAFI® and BAT group respectively (P=0.003). More patients assigned to JAKAFI® group also demonstrated 50% or more improvement in the Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF) 14-item total symptom score, at week 32 compared to BAT (49% vs 5%). Thromboembolic events occurred in one patient assigned to the JAKAFI® group as compared to six patients in the BAT group. The authors concluded that JAKAFI® may represent a new option for treating high risk patients with Polycythemia Vera, who are refractory or intolerant of Hydroxyurea. JAKAFI® is superior to Best Available Therapy (BAT) in controlling hematocrit without phlebotomies as well as Splenic Volume. Further, JAKAFI® is also effective in improving P. Vera associated symptoms. Verstovsek S, Kiladjian J, Griesshammer M, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 7026)
JAKAFI® is a potent JAK1 and JAK2 inhibitor and exerts its mechanism of action by targeting and inhibiting the dysregulated JAK2-STAT signaling pathway. The RESPONSE trial is a phase III prospective randomized study in which patients with P. Vera, who were refractory or intolerant of Hydroxyurea were randomized to receive JAKAFI® 10 mg PO, BID (N=110) or Best Available Therapy (BAT), which consisted of investigator choice of monotherapy or observation only (N=112). Eligible patients were phlebotomy dependent and had splenomegaly (> 450 cubic cm). Patients receiving BAT were allowed to cross over to JAKAFI® group from week 32 onwards. The primary endpoint of this study (composite primary endpoint) was the proportion of patients whose hematocrit was controlled without phlebotomy and whose spleen volume was reduced by 35% or more from baseline, as assessed by MRI imaging at 32 weeks. Secondary endpoints included durable response, Complete Hematological Remission and safety. The primary analysis was conducted when all patients reached week 48 or discontinued therapy. The proportion of patients in the JAKAFI® group who achieved the composite primary endpoint was 21% compared to 1% in the BAT group (P < 0.0001) and 91% in the JAKAFI® group maintained their response at week 48. Seventy seven percent (77%) of the patients in the JAKAFI® group achieved at least one of the two major components of the composite primary endpoint. Put another way, 60% of the patients in the JAKAFI® arm were able to achieve the target hematocrit level in the absence of phlebotomy, compared to 20% in the BAT group. Reduction in the spleen volume by 35% or more was noted in 38% of the patients in the JAKAFI® group compared to 1% in the BAT group. Complete Hematological Remission defined as continuous hematocrit below 45%, as well as normal white blood cells and platelets counts, was achieved in 24% and 9% of patients in JAKAFI® and BAT group respectively (P=0.003). More patients assigned to JAKAFI® group also demonstrated 50% or more improvement in the Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF) 14-item total symptom score, at week 32 compared to BAT (49% vs 5%). Thromboembolic events occurred in one patient assigned to the JAKAFI® group as compared to six patients in the BAT group. The authors concluded that JAKAFI® may represent a new option for treating high risk patients with Polycythemia Vera, who are refractory or intolerant of Hydroxyurea. JAKAFI® is superior to Best Available Therapy (BAT) in controlling hematocrit without phlebotomies as well as Splenic Volume. Further, JAKAFI® is also effective in improving P. Vera associated symptoms. Verstovsek S, Kiladjian J, Griesshammer M, et al. J Clin Oncol 32:5s, 2014 (suppl; abstr 7026)
Molecular Testing for Selection of Patients with Lung Cancer for Epidermal Growth Factor Receptor and Anaplastic Lymphoma Kinase Tyrosine Kinase Inhibitors American Society of Clinical Oncology Endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology Guideline
SUMMARY: There is now growing body of evidence suggesting superior outcomes when advanced NSCLC patients with specific genomic alterations receive targeted therapies. Following review of 127 studies by experts and input from a scientific advisory panel, The College of American Pathologists (CAP), the International Association for the Study of Lung Cancer (IASLC), and the Association for Molecular Pathology (AMP) offered evidence-based recommendations for the molecular analysis of lung cancers for Epidermal Growth Factor Receptor (EGFR ) mutations and Anaplastic Lymphoma Kinase (ALK) rearrangements, thereby selecting patients with lung cancer, for treatment with EGFR and ALK tyrosine kinase inhibitors. The ASCO review panel has endorsed these guidelines which specifically address the following questions:
1) Which patients should be tested for EGFR mutations and ALK rearrangements?
EGFR or ALK testing is recommended for all patients with advanced lung adenocarcinoma or tumors with an adenocarcinoma component, irrespective of clinical characteristics such as smoking history, sex, race, or other clinical factors. Tumor samples of other histologies for which an adenocarcinoma component cannot be excluded because of sampling, can be considered for testing, particularly if clinical criteria are suggestive (eg, younger age, lack of smoking history). Both primary tumors and metastatic lesions are suitable for testing. When fully excised lung cancer specimens are available, EGFR and ALK testing is not recommended in lung cancers that lack any adenocarcinoma component, such as pure squamous cell carcinomas, pure small-cell carcinomas, or large-cell carcinomas lacking IHC (ImmunoHistoChemistry) evidence of adenocarcinoma differentiation.
2) When should a patient specimen be tested for EGFR mutation or ALK rearrangement?
Testing should be ordered at the time of diagnosis of advanced disease or recurrence. For patients with earlier stage disease who undergo surgical resection, testing at the time of diagnosis is encouraged so that molecular information is available to an oncologist at the time of recurrence, for a subset of patients who subsequently experience recurrence. Tissue should be prioritized for EGFR and ALK testing.
 3) How rapidly should test results be available?
3) How rapidly should test results be available?
Laboratory turnaround times of 5 to 10 working days (2 weeks) for EGFR and ALK results are recommended.
4) How should specimens be processed for EGFR mutation testing?
Pathologists should use Formalin-Fixed, Paraffin-Embedded (FFPE) specimens or fresh frozen or alcohol-fixed specimens for PCR based EGFR mutation tests. EGFR and ALK testing can be performed with cytology samples, with cell blocks being preferred over smear preparations.
5) How should EGFR testing be performed?
EGFR testing should detect mutations in samples composed of as few as 50% tumor cells, although sensitivity to detect mutations in samples containing > 10% tumor cells is strongly encouraged. Sensitizing EGFR mutations with a population frequency of at least 1% should be reported. IHC for total EGFR as well as EGFR copy number analysis by FISH (Fluorescence In Situ Hybridization) is not recommended.
6) What is the role of KRAS analysis in selecting patients for targeted therapy with EGFR TKIs?
KRAS mutations are common (30%) in lung adenocarcinomas and mutually exclusive with EGFR and ALK. Testing for KRAS may be performed initially to exclude KRAS mutated tumors from EGFR and ALK testing but KRAS mutation testing is not recommended as a sole determinant of EGFR-targeted therapy.
7) What additional testing considerations are important in the setting of secondary or acquired EGFR TKI resistance?
If a laboratory performs testing on specimens from patients with acquired resistance to EGFR kinase inhibitors, such tests should be able to detect the secondary EGFR T790M mutation in as few as 5% of cells.
8) What methods should be used for ALK testing?
ALK FISH assay using dual labeled break-apart probes should be used for selecting patients for ALK TKI therapy. ALK IHC, if carefully validated, may be considered as a screening methodology to select specimens for ALK FISH testing. RT-PCR (Reverse Transcription–Polymerase Chain Reaction) is not recommended as an alternative to FISH, for selecting patients for ALK inhibitor therapy.
9) Are other molecular markers suitable for testing in lung cancer?
Testing for EGFR should be prioritized over other molecular markers in lung adenocarcinoma followed by testing for ALK. Testing for ROS1 and RET rearrangements may soon become a part of the guidelines.
10) How should molecular testing of lung adenocarcinomas be implemented and operationalized?
Pathology departments should establish a process wherein tissue (blocks or unstained slides) is sent to outside molecular laboratories within 3 days of receiving a request and to in house molecular laboratories within 24 hours. Results should be available within 2 weeks and reported in a format that is easily understood by oncologists and nonspecialist pathologists.
Leighl NB, Rekhtman N, Biermann WA, et al. J Clin Oncol 2014;32:3673-3679
JAKAFI® (Ruxolitinib)
The FDA on December 4, 2014 approved JAKAFI® for the treatment of patients with Polycythemia Vera (PV) who have had an inadequate response to or are intolerant of Hydroxyurea (HU). JAKAFI® is a product of Incyte Corporation.
BLINCYTO® (Blinatumomab)
The FDA on December 3, 2014 granted accelerated approval for BLINCYTO® for the treatment of Philadelphia chromosome-negative relapsed or refractory B-cell precursor Acute Lymphoblastic Leukemia (R/R ALL). BLINCYTO® is a product of Amgen Inc.
AVASTIN® (Bevacizumab)
The FDA on November 14, 2014 approved AVASTIN® in combination with paclitaxel, pegylated liposomal doxorubicin, or topotecan for the treatment of patients with platinum-resistant, recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer. AVASTIN® is a product of Genentech, Inc.
Sentinel Lymph Node Biopsy for Patients with Early-Stage Breast Cancer American Society of Clinical Oncology Clinical Practice Guideline Update
SUMMARY: Breast cancer is the most common cancer among women in the US and about 1 in 8 women (12%) will develop invasive breast cancer during their lifetime. Approximately, 233,000 new cases of invasive breast cancer will be diagnosed in 2014 and 40,000 women will die of the disease. Surgical resection of the axillary lymph nodes in addition to potentially removing cancer that may have spread, also facilitates staging of breast cancer. 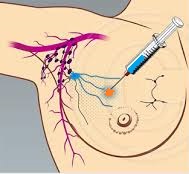 The sentinel node is the first lymph node(s) to which cancer cells are most likely to metastasize from a primary tumor. With the introduction of intraoperative lymphatic mapping in the 1990s, Sentinel Lymph Node Biopsy (SLNB) has gained general acceptance and is the preferred procedure in appropriate circumstances. Unlike Axillary Lymph Node Dissection (ALND), SLNB is associated with a lower incidence of Lymphedema, seroma at the surgery site, paresthesias and restriction of joint movement. Nine randomized clinical trials have not shown any difference in mortality among patients who underwent ALND or SLNB for either lymph node metastases or negative sentinel lymph nodes, validating Sentinel Lymph Node Biopsy (SLNB). The American Society of Clinical Oncology (ASCO) first published guidelines on the use of SLNB for patients with early stage breast cancer in 2005, based on one randomized clinical trial. Since then, additional information from 9 randomized clinical trials and13 cohort studies pertinent to SLNB and ALND has resulted in this ASCO Clinical Practice Guideline Update.
The sentinel node is the first lymph node(s) to which cancer cells are most likely to metastasize from a primary tumor. With the introduction of intraoperative lymphatic mapping in the 1990s, Sentinel Lymph Node Biopsy (SLNB) has gained general acceptance and is the preferred procedure in appropriate circumstances. Unlike Axillary Lymph Node Dissection (ALND), SLNB is associated with a lower incidence of Lymphedema, seroma at the surgery site, paresthesias and restriction of joint movement. Nine randomized clinical trials have not shown any difference in mortality among patients who underwent ALND or SLNB for either lymph node metastases or negative sentinel lymph nodes, validating Sentinel Lymph Node Biopsy (SLNB). The American Society of Clinical Oncology (ASCO) first published guidelines on the use of SLNB for patients with early stage breast cancer in 2005, based on one randomized clinical trial. Since then, additional information from 9 randomized clinical trials and13 cohort studies pertinent to SLNB and ALND has resulted in this ASCO Clinical Practice Guideline Update.
The following recommendations were made by the American Society of Clinical Oncology panel of experts:
1) Women without sentinel lymph node (SLN) metastases should not undergo Axillary Lymph Node Dissection (ALND).
2) Women with one to two metastatic SLNs planning to undergo breast conserving surgery with whole breast radiotherapy should not undergo ALND (in most cases).
3) Women with SLN metastases who will undergo mastectomy should be offered ALND.
4) Women with operable breast cancer and multicentric tumors, those with ductal carcinoma in situ (DCIS) who will undergo mastectomy, those who previously underwent breast and/or axillary surgery and those who received preoperative/neoadjuvant systemic therapy, may be offered SLNB.
5) Women who have large or locally advanced invasive breast cancer (tumor size T3/T4), inflammatory breast cancer, or DCIS (when breast-conserving surgery is planned) or are pregnant, should not undergo SLNB.
Lyman GH, Temin S, Edge SB, et al. J Clin Oncol 2014;32:1365-1383
